Effects of pH value and temperature on the initiation, promotion, inhibition and direct reaction rate constants of natural organic matter in ozonation†
Abstract
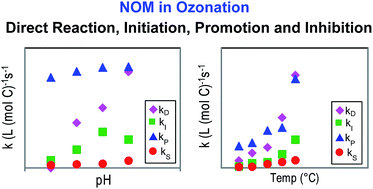
The effects of pH value and temperature on the initiation (kI), promotion (kP), inhibition (kS) and direct ozone reaction (kD) rate constants of natural organic matter (NOM) in water ozonation were investigated in this study. These rate constants were determined using a newly developed method that integrates the classical Rct concept, the transient steady-state hydroxyl radical (˙OH) concentration model and the pseudo first-order ozone decomposition model. Suwannee River fulvic acid (SRFA) was selected as the model NOM. Our results showed that (1) the variation of pH value from 6.5–8.0 had little influence on kP; while kI showed a peak value at pH 7.5, and kS and kD increased with increasing pH, (2) at room temperature, the value of kD is 2.7–5.8 times higher than kI and that of kP is 14–31 times higher than kS at pH 6.5–8.0, indicating that direct ozone reaction and promotion reaction are the dominant pathways for SRFA to react with ozone and ˙OH, respectively, and (3) all rate constants showed a strong dependency on temperature and the activation energies for initiation, promotion, inhibition and direct ozone reaction were determined to be 55.3, 25.6, 50.1 and 49.1 kJ mol−1, respectively. Functional groups in NOM that are potentially responsible for these reactions were discussed. Our results provide deeper insight into the reactions between NOM and ozone/˙OH, and the removal of micropollutants in the ozonation process can be evaluated using the rate constants determined in this study.


 Please wait while we load your content...
Please wait while we load your content...