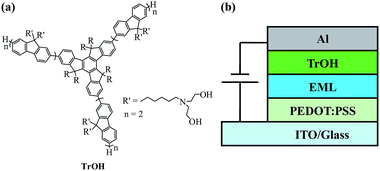
Efficient phosphorescent polymer light-emitting devices using a conjugated starburst macromolecule as a cathode interlayer
Xin-Wen Zhanga,
Zhen-Feng Leia,
Yue-Hua Chena,
Ke-Yu Chena,
Wei-Dong Xua,
Lin Haoa,
Qu-Li Fana,
Wen-Yong Lai*ab and
Wei Huang*ab
aKey Laboratory for Organic Electronics and Information Displays, Institute of Advanced Materials, Jiangsu National Synergetic Innovation Center for Advanced Materials, Nanjing University of Posts & Telecommunications, Nanjing 210023, China. E-mail: iamwylai@njupt.edu.cn
bKey Laboratory of Flexible Electronics, Institute of Advanced Materials, Jiangsu National Synergetic Innovation Center for Advanced Materials, Nanjing Tech University, Nanjing 211816, China. E-mail: wei-huang@njtech.edu.cn
First published on 14th January 2016
Abstract
We present the results of a systematic study of a conjugated starburst macromolecule TrOH cathode interlayer produced by solution-processing for the fabrication of highly efficient multilayered phosphorescent polymer light-emitting devices (PhPLEDs). It was found that the performance of the PhPLEDs was strongly dependent on the solvent composition of the TrOH coatings. The devices with the interlayer deposited from the mixed-solvent of water and ethanol showed much better device performance than that of the device with ethanol as solvent. From the impedance spectra of the devices and UV-vis absorption spectra of the emission layers (EMLs) treated by ethanol or mixed-solvent, the variation in device performance is mainly attributed to washing out the electron transport material in the EML due to the rinse effect. The erosion of the EML could be greatly suppressed by adding an appropriate amount of water into ethanol. The optimized green device with a maximum luminous efficiency of 23.4 cd A−1 and an external quantum efficiency of 6.7% was obtained when the ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio approached 7
water ratio approached 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v). The peak efficiency of 14.6 cd A−1 was also achieved in a single emissive layer white PhPLED with a TrOH interlayer. In order to further understand the effect of the TrOH interlayer deposited from different solutions on the device performance, atomic force microscopy and contact angles were used to investigate the surface properties of the TrOH interlayer. The results indicate that the interfacial morphology is mainly controlled by the wetting characteristics of the EML and TrOH solution. The inferior device performance can be ascribed to the discontinuous TrOH film when the amount of water in TrOH solution is above 40%, which leads to the subdued electron injection from cathode into the EML.
3 (v/v). The peak efficiency of 14.6 cd A−1 was also achieved in a single emissive layer white PhPLED with a TrOH interlayer. In order to further understand the effect of the TrOH interlayer deposited from different solutions on the device performance, atomic force microscopy and contact angles were used to investigate the surface properties of the TrOH interlayer. The results indicate that the interfacial morphology is mainly controlled by the wetting characteristics of the EML and TrOH solution. The inferior device performance can be ascribed to the discontinuous TrOH film when the amount of water in TrOH solution is above 40%, which leads to the subdued electron injection from cathode into the EML.
1. Introduction
Polymer light-emitting devices (PLEDs) have been drawing much attention because of their great potential in cost-effective and large-area displays and lighting applications.1–3 In particular, phosphorescent PLEDs (PhPLEDs) based on phosphorescent dopants dispersed in a polymeric host are highly attractive due to their potential for achieving unity internal quantum efficiency through harvesting both singlet and triplet excitons.4 So far, vacuum evaporated phosphorescent devices based on small molecules outperform the solution processed PhPLEDs because thermal evaporation allows for stacking various functional layers to tune the balance of electrons and holes in the emitting layer (EML) while solution-processing is ruled by solvent limitations. Though a device with a single-layer structure is encouraged because of its simplicity, it often shows low luminous efficiency and high turn-on voltage due to unbalanced carrier injection from electrodes into the EML. In order to achieve high-efficiency and low operational voltage, multilayered structures with insertion of appropriate hole-injection/transport layers (HILs/HTLs) or electron-injection/transport layers (EILs/ETLs) at the cathode and anode interfaces are desired. To facilitate the injection of holes, hole injection materials such as poly(3,4-ethylenedioxythiophene):poly(styrenesulfonic acid) (PEDOT:PSS),5–7 metal oxides (MoOx, WO3, V2O5 and NiOx),8–11 graphene oxide12 and cross-linkable molecules13–15 have been selected to act as the solution-processed hole injection layer. Simultaneously, for the purpose of facilitating electron injection, vacuum-deposited low work function metals (Ca or Ba),16 ultrathin EILs of alkali salts (LiF, CsF and Cs2CO3) and ETLs/EILs such as 1,3,5-tris[N-(phenyl)benzimidazole]-benzene (TPBi)/LiF, 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline (BCP)/LiF and TPBi/CsF17–19 are developed to reduce operational voltage and increase luminous efficiency. However, these low work function metals are sensitive to oxygen and humidity, thus deteriorating device operational stability. Additionally, ETLs/EILs by vacuum deposition can lead to high cost and complexity of the devices,20 which are incompatible with low-cost, large-area manufacture technology such as screen printing, ink-jet printing and roll-to-roll coating.21–23To overcome such drawbacks, it is desirable to achieve stable and soluble electron injection/transport materials with orthogonal solubilities compared to that of the EML. Consequently, the coating solvent for the EIL will not dissolve the light-emitting layer. Recently, great efforts have been made to develop new solution-processed electron injection/transport materials with orthogonal solubilities,24–32 in combination with high efficiency and a low operating voltage. One approach is to use solution-processable n-type metal oxides such as ZnO, TiO2 and ZrO224–27 as efficient EILs in the inverted PLEDs. In general, these metal oxide films are fabricated by solution-deposition techniques onto indium tin oxide (ITO) substrates using metal oxide precursors. A high-temperature treatment is often needed to convert the precursor solution to the desired metal oxide, which is incompatible with plastic substrates in printing manufacturing.28 Another approach to enhance electron injection is to use strongly polarized polymers as electron injection layers in PLEDs.29–31,33–37 These polarized polymers can be dissolved in polar solvents such as water and alcohol, and allow fabrication of multilayered device structures by solution-processing methods that do not perturb underlying layers. Inserting a layer of polarized polymers between the EML and cathode can result in efficient electron injection even from high-work function metals (like Au, Ag or Al). One possible explanation for lowering electron injection barriers originates from the charged or polar groups on the side chains, which can generate a positive interfacial dipole between cathode and EML, leading to a raised vacuum level close to that of the electrode.38 Though polarized polymers have excellent film-forming properties and device performance, they suffer from problems such as batch-to-batch variation in terms of molecular weight and polydispersity, difficulty in purification, limited solubility and poor device reproducibility. Recently, our group developed a water/alcohol soluble conjugated starburst macromolecule TrOH using as efficient EIL for PLEDs.39,40 Multilayered devices were fabricated by solution-processing that do not perturb underlying layers. When a thin TrOH layer was inserted between the EML and Al cathode, the performance of the device was even better than that of the device using Ca as cathode. Despite the excellent performance of TrOH based PLEDs, the application of TrOH in phosphorescent devices has not been systematically investigated.
In this work, we present a systematic study in highly efficient multilayered PhPLEDs by solution-processing using the conjugated starburst macromolecule TrOH as a cathode interlayer. We found that the performance of the PhPLEDs was strongly dependent on the solvent composition for TrOH coating. The electron transport material 1,3-bis[(4-tert-butylphenyl)-1,3,4-oxadiazolyl] phenylene (OXD-7) in the EMLs was eroded by ethanol solvent during the processing of TrOH layer, which is an important reason for deterioration of the device performance. Highly efficient PhPLEDs were achieved by adding an appropriate amount of water into ethanol as mixed-solvent to suppress the erosion of the EMLs. Our results have offered a new approach to easily fabricate low-cost and high-efficiency PhPLEDs with a multilayer structure by solution-processing.
2. Experimental
The hole-injection material of poly(3,4-ethylenedioxythiophene):poly(styrenesulfonic acid) (PEDOT:PSS, AI4083) was purchased from H. C. Starck Inc. The polymer host of poly(N-vinylcarbazole) (PVK, average Mw = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from Sigma-Aldrich. The electron-transporting material of OXD-7 and the phosphorescent dopants of bis[(4,6-difluorophenyl)-pyridinato-N,C2](picolinate)iridium(III) (FIrpic), tris(2-4(4-toltyl)phenylpyridine)iridium [Ir(mppy)3] and Tris(2-phenylquinoline-C2,N′)iridium(III) [Ir(2-phq)3] were purchased from Nichem Fine Technology Co. Ltd. All the above-mentioned materials were used as-received without further purification. The conjugated starburst macromolecule TrOH was synthesized in our laboratory. The synthesis procedures were described elsewhere.39 The chemical structure of TrOH is shown in Fig. 1(a). The number average molecular weight of precursor polymer estimated by gel permeation chromatography using polystyrene as the standard and THF as the eluent is 18
000) was purchased from Sigma-Aldrich. The electron-transporting material of OXD-7 and the phosphorescent dopants of bis[(4,6-difluorophenyl)-pyridinato-N,C2](picolinate)iridium(III) (FIrpic), tris(2-4(4-toltyl)phenylpyridine)iridium [Ir(mppy)3] and Tris(2-phenylquinoline-C2,N′)iridium(III) [Ir(2-phq)3] were purchased from Nichem Fine Technology Co. Ltd. All the above-mentioned materials were used as-received without further purification. The conjugated starburst macromolecule TrOH was synthesized in our laboratory. The synthesis procedures were described elsewhere.39 The chemical structure of TrOH is shown in Fig. 1(a). The number average molecular weight of precursor polymer estimated by gel permeation chromatography using polystyrene as the standard and THF as the eluent is 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (with a polydispersity of 1.8).
000 (with a polydispersity of 1.8).
In the experiment, the patterned ITO glass substrates were ultrasonically cleaned with detergent, alcohol and acetone, deionized water and then dried at 120 °C in a vacuum oven for more than one hour. After ultraviolet (UV)-ozone treating for 4 min, a 40 nm PEDOT:PSS was spin-coated on the ITO substrate and dried at 120 °C in a vacuum oven for 15 min to extract the residual solvent. The emitting layer was then deposited on top of PEDOT:PSS layer, by casting from a chlorobenzene solution containing 4 wt% Ir(mppy)3 doped into a PVK (60 wt%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OXD-7 (40 wt%) matrix, followed by thermal annealing at 100 °C for 20 min. The thickness of the EMLs is about 80 nm. Afterward, an alcohol/water soluble cathode interlayer of TrOH was spin-coated on EML from 0.5 mg mL−1 ethanol or water/ethanol (v/v) solutions. Subsequently, the samples were transferred to a thermal evaporator chamber. The Al (100 nm) cathode was deposited by thermal evaporation under a pressure of 5 × 10−4 Pa. Meanwhile, a reference OLED with a structure of ITO/PEDOT:PSS/EML/Al was also prepared for comparison. For the white device, 10 wt% FIrpic and 0.5 wt% Ir(2-phq)3 co-doped into the PVK
OXD-7 (40 wt%) matrix, followed by thermal annealing at 100 °C for 20 min. The thickness of the EMLs is about 80 nm. Afterward, an alcohol/water soluble cathode interlayer of TrOH was spin-coated on EML from 0.5 mg mL−1 ethanol or water/ethanol (v/v) solutions. Subsequently, the samples were transferred to a thermal evaporator chamber. The Al (100 nm) cathode was deposited by thermal evaporation under a pressure of 5 × 10−4 Pa. Meanwhile, a reference OLED with a structure of ITO/PEDOT:PSS/EML/Al was also prepared for comparison. For the white device, 10 wt% FIrpic and 0.5 wt% Ir(2-phq)3 co-doped into the PVK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OXD-7 matrix. The structure of the device is shown in Fig. 1(b). For UV-vis absorption spectra measurement, the neat EML and equivalent films washed by ethanol and ethanol
OXD-7 matrix. The structure of the device is shown in Fig. 1(b). For UV-vis absorption spectra measurement, the neat EML and equivalent films washed by ethanol and ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (7
water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) were fabricated, respectively, and the conditions of spin-coating and thermal annealing were similar to those used for green PLEDs fabrication. The emission area of the devices is 13.5 mm2.
3) were fabricated, respectively, and the conditions of spin-coating and thermal annealing were similar to those used for green PLEDs fabrication. The emission area of the devices is 13.5 mm2.
The luminance–current–voltage characteristics of the devices were recorded using a combination of a Keithley source-meter (model 2602) and a calibrated luminance meter. Electroluminescence (EL) spectra and Commission International de l'Eclairage (CIE) coordinates were obtained using a spectra-scan PR655 spectrophotometer. The thickness of the organic films was measured with a spectroscopic ellipsometry (α-SE, J. A. Wollam Co. Inc.). For photovoltaic measurements, the photocurrent–voltage characteristics were recorded using a computer-controlled Keithley 2400 source meter and a solar simulator (Newport 91160) under AM 1.5G condition at 100 mW cm−2. The surface morphology of the films was investigated by atomic force microscopy (AFM, Bruker Dimension Icon). Absorption spectra were measured using a spectrophotometer (SHIMADZU UV-3600). The contact angles of the films were measured with a CAM 200 (KSV Instrument LID.) and the photos were taken with a BASLER A602f-2 camera. The impedance spectroscopy measurements for the fabricated devices were performed using an impedance analyzer (Wayne Kerr 6505B) at frequencies ranging from 20 Hz to 5 MHz, with a 200 mV perturbation oscillation signal to maintain the linearity of the response. All the devices were characterized without encapsulation, and all the measurements were carried out under ambient condition at room temperature.
3. Results and discussion
To estimate the effect of the cathode interlayer on the device performance, solution-processed green PhPLEDs with a structure of ITO/PEDOT:PSS/EML/TrOH/Al were examined. In these devices, green-emissive Ir(mppy)3 was doped into PVK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OXD-7 (6
OXD-7 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) matrix to form the EML with a doping concentration of 4 wt%. The TrOH was deposited from ethanol or mixtures of ethanol and water with various ethanol
4) matrix to form the EML with a doping concentration of 4 wt%. The TrOH was deposited from ethanol or mixtures of ethanol and water with various ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water of 9
water of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8
1, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 7
2, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 6
3, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 4
4 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (v/v). For comparison, the device without an interlayer was also fabricated. The performance of the solution-processed green phosphorescent devices is summarized in Table 1. It can be seen that the performance of the devices with a TrOH interlayer is much better than that of the device without it. On the other hand, it is interesting to note that the performance of these devices is highly dependent on the solution composition for TrOH coating. The device performance improves remarkably when TrOH was deposited from a mixed-solvent of ethanol
6 (v/v). For comparison, the device without an interlayer was also fabricated. The performance of the solution-processed green phosphorescent devices is summarized in Table 1. It can be seen that the performance of the devices with a TrOH interlayer is much better than that of the device without it. On the other hand, it is interesting to note that the performance of these devices is highly dependent on the solution composition for TrOH coating. The device performance improves remarkably when TrOH was deposited from a mixed-solvent of ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water. As seen from Fig. 2, the current densities of the devices decrease significantly with the increasing water amount in the solutions. At the specific ethanol
water. As seen from Fig. 2, the current densities of the devices decrease significantly with the increasing water amount in the solutions. At the specific ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio (7
water ratio (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), the device exhibited the best performance, achieving a maximum luminance of 18
3), the device exhibited the best performance, achieving a maximum luminance of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 cd m−2 and current efficiency of 23.4 cd A−1, respectively, which are higher than those observed with TrOH deposited from pure ethanol (3246 cd m−2 and 5.1 cd A−1), and are almost an order of magnitude higher than those with Al (762 cd m−2 and 2.5 cd A−1). Most important to note that the efficiency-brightness roll-off was also improved for the devices with the TrOH interlayer deposited from mixed-solvent. With the brightness rising up to 3000 cd m−2, the efficiency roll-off is about 73% for the device with TrOH interlayer deposited from ethanol, and the efficiency roll-off is less than 5% at the same luminance for the device with TrOH interlayer deposited from ethanol
050 cd m−2 and current efficiency of 23.4 cd A−1, respectively, which are higher than those observed with TrOH deposited from pure ethanol (3246 cd m−2 and 5.1 cd A−1), and are almost an order of magnitude higher than those with Al (762 cd m−2 and 2.5 cd A−1). Most important to note that the efficiency-brightness roll-off was also improved for the devices with the TrOH interlayer deposited from mixed-solvent. With the brightness rising up to 3000 cd m−2, the efficiency roll-off is about 73% for the device with TrOH interlayer deposited from ethanol, and the efficiency roll-off is less than 5% at the same luminance for the device with TrOH interlayer deposited from ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (7
water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Commonly, the efficiency roll-off at high brightness in phosphorescent device was mainly attributed to triplet–triplet annihilation (TTA) or/and triplet–polaron quenching (TPQ) in the EML.41 Charge carrier imbalance is usually an important factor for the efficiency roll-off behavior in phosphorescent device.42 The solvent composition used for the TrOH interlayer deposition may change charge carriers injection and transporting characteristics of the device. A detailed discussion will be presented later. The turn-on voltage is also significantly reduced compared to the device with Al cathode. For example, the turn-on voltage (defined as the voltage at 1 cd m−2) of the device (ethanol
3). Commonly, the efficiency roll-off at high brightness in phosphorescent device was mainly attributed to triplet–triplet annihilation (TTA) or/and triplet–polaron quenching (TPQ) in the EML.41 Charge carrier imbalance is usually an important factor for the efficiency roll-off behavior in phosphorescent device.42 The solvent composition used for the TrOH interlayer deposition may change charge carriers injection and transporting characteristics of the device. A detailed discussion will be presented later. The turn-on voltage is also significantly reduced compared to the device with Al cathode. For example, the turn-on voltage (defined as the voltage at 1 cd m−2) of the device (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water = 7
water = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) is 4.96 V, which is reduced from 11.40 V of the control device. Additionally, it should be noted that the turn-on voltages of the devices with TrOH interlayer deposited from mixed-solvent are very close, indicating electron injection barrier height of these devices are similar. These results suggest that the introduction of TrOH in the devices can indeed improve the electron-injection due to reduced electron injection barrier height,39 which is supported by built-in potential measurement shown in Fig. 3. As deduced from the current density–voltage under illumination from solar simulator, the built-in across the device shift from 1.0 V for the Al device to 1.6 V for the TrOH (ethanol
3) is 4.96 V, which is reduced from 11.40 V of the control device. Additionally, it should be noted that the turn-on voltages of the devices with TrOH interlayer deposited from mixed-solvent are very close, indicating electron injection barrier height of these devices are similar. These results suggest that the introduction of TrOH in the devices can indeed improve the electron-injection due to reduced electron injection barrier height,39 which is supported by built-in potential measurement shown in Fig. 3. As deduced from the current density–voltage under illumination from solar simulator, the built-in across the device shift from 1.0 V for the Al device to 1.6 V for the TrOH (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water = 7
water = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)/Al device, indicating that electron injection barrier height of the device with the TrOH interlayer is reduced.
3)/Al device, indicating that electron injection barrier height of the device with the TrOH interlayer is reduced.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (v/v) ratios
water (v/v) ratios
TrOH (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water) water) |
Von (V) | Lmax (cd m−2)/V (V) | LEmax (cd A−1) | EQEmax (%) |
|---|---|---|---|---|
| No | 11.40 | 762/20 | 2.5 | — |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
5.79 | 3246/14.0 | 5.1 | 1.5 |
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4.97 | 7029/14.9 | 11.3 | 3.2 |
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
4.76 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 666/15.5 666/15.5 |
18.9 | 5.4 |
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
4.96 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050/16.5 050/16.5 |
23.4 | 6.7 |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
4.98 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 490/16.0 490/16.0 |
22.2 | 6.4 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
5.09 | 6830/16.5 | 18.7 | 5.4 |
 | ||
| Fig. 2 Comparison of the device characteristics in the solution-processed green PhPLEDs: (a) current density–voltage, (b) luminance–voltage and (c) current efficiency–luminance. | ||
 | ||
Fig. 3 Photovoltaic characteristics of the solution-processed green phosphorescent OLEDs with Al and TrOH (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water = 7 water = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)/Al cathode. 3)/Al cathode. | ||
Changing the ratio between water and methanol can significantly affect on the device performance of the green PhPLEDs. It is notable that the TrOH (ethanol)/Al device shows a maximal current density over other devices, but an improvement is not achieved in the luminance and efficiency. In addition, the performance of the device is improved gradually when the content of water in the TrOH solution increases from 0 to 30%, but it begins to go off when the amount of water is above 30%. Furthermore, it should be noted that the turn-on voltage of the device with the TrOH interlayer deposited from ethanol is higher than that of the device with the TrOH interlayer deposited from mixed-solvent. These will be discussed later.
In order to get insight into the strong dependence of device performance on the solvent composition, we use ac impedance spectroscopy to investigate the electrical properties of the devices, which is a powerful technique for investigating the electrical properties of materials and their interfaces.43–45 Fig. 4 shows the measured and simulated Cole–Cole plots of the green PhPLEDs at zero bias. It is interesting to see the significant difference among impedance spectra of the devices with TrOH deposited from different solutions. The frequency increases from right to left for the measured data, and the horizontal and vertical axes of the plots represent the real part Re(Z) and imaginary part Im(Z) of the complex impedance of the devices, respectively. It is clear that the impedance spectra show single-semicircle impedance characteristics in the Cole–Cole plots, with the radius of the semicircle increasing abruptly with increasing water amount in the TrOH solutions. Since the radius is related to the total resistance of the device, the impedance increases with increasing water amount in the TrOH solutions. Thus, the equivalent circuit for the devices can be considered as a single parallel resistor Rp and capacitor Cp network with a series resistance Rs, as shown in the inset of the Fig. 4.45 The series resistance Rs is associated with the contribution from the contact at the electrode interface. The parallel resistance Rp in the parallel RC network is associated with the layer resistance (EML), while Cp is correlated with the geometric capacitance of the EML. As also shown in Fig. 4, by carefully adjusting the parameters (Rs, Rp, Cp), each curve in the Cole–Cole plot can be well reproduced using the equivalent circuit. The variation of the obtained parameters (Rp and Cp) by fitting the measured Cole–Cole plots with the content of water in the TrOH solutions is shown in Fig. 5. The parallel capacitor Cp decreases rapidly from 7.35 to 4.92 nF by increasing the water content in the TrOH solutions from 0% to 60%, while the parallel resistance Rp increases with an increment of water contents. As we know, the geometric capacitor is defined as C = ε0εA/d, in which ε is the dielectric constant of EML, ε0 is the vacuum permittivity, A is the contact area, and d is the thickness.43 Therefore, the capacitance reduction with the content of water can be ascribed to the increasing thickness of EML. In other words, the EML is partially eroded by the solvent of upper layer during spin-coating. The erosion of EML by ethanol can be gradually suppressed by adding water into ethanol. Therefore, the impedance of the device increases with increasing water amount in the TrOH solutions. By assuming an equal dielectric constant of 3 for all components,46 the thickness of the residual EML after washed by TrOH ethanol solution is estimated to be about 49 nm from the Cp value. The thickness of the EML washed out by ethanol is about 31 nm. It is not difficult to understand that the TrOH (ethanol)/Al device shows a maximal current density over other devices.
 | ||
| Fig. 5 The variation of the obtained parallel capacitance Cp and parallel resistance Rp with the content of water in the TrOH solutions for the green PhPLEDs studied. | ||
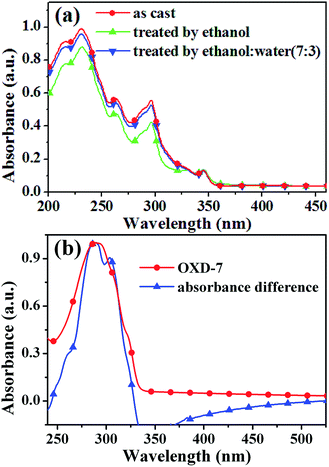
To further confirm the possible eroded compositions of the EML, UV-vis absorption spectra of neat EML and solvent treated EML were measured. In these studies, neat EML and equivalent films washed by ethanol and ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (7
water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) were fabricated, respectively. Fig. 6(a) shows the absorbance spectra of the EML films with and without a solvent rinse. It is found that the absorption is obviously decreased after ethanol treatment, but the distinct difference is not observed with a mixed-solvent (ethanol/water). Furthermore, the spectral difference between the neat film and those treated by ethanol is very similar to the absorption spectra of OXD-7 (Fig. 6(b)). From the results as mentioned above, we can conclude that small molecule OXD-7 is wasted out partially from the EMLs by ethanol during the processing of TrOH. Such a compositional variation in the active layer is likely responsible for the inferior performance of the device. The erosion of OXD-7 by ethanol can be greatly suppressed by adding an appropriate amount of water into ethanol.
3) were fabricated, respectively. Fig. 6(a) shows the absorbance spectra of the EML films with and without a solvent rinse. It is found that the absorption is obviously decreased after ethanol treatment, but the distinct difference is not observed with a mixed-solvent (ethanol/water). Furthermore, the spectral difference between the neat film and those treated by ethanol is very similar to the absorption spectra of OXD-7 (Fig. 6(b)). From the results as mentioned above, we can conclude that small molecule OXD-7 is wasted out partially from the EMLs by ethanol during the processing of TrOH. Such a compositional variation in the active layer is likely responsible for the inferior performance of the device. The erosion of OXD-7 by ethanol can be greatly suppressed by adding an appropriate amount of water into ethanol.
Additionally, it is noted that the performance of the devices gradually worsens when the amount of water is above 30%. According to the early reports,39,47 the morphology of solution-processed interlayer can significantly affect the charge injection property, which is directly related to the device performance. To further understand the effect of the TrOH interlayer deposited from different solutions on the device performance, the AFM images of TrOH layers on top of EMLs were measured. Fig. 7 shows the AFM images of the solution-processed EML and TrOH films spin-coated on top of the EMLs from ethanol or ethanol/water solvents. These experimental results show that the surface topography of TrOH films can be altered dramatically by the solvent used for spin-coating. As shown in Fig. 7(a), the root-mean-square (RMS) roughness of the EML is only 0.39 nm. However, the quality of TrOH film is much poorer when formed from ethanol and its RMS roughness is 3.24 nm. The rougher surface can be attributed to the erosion of EML by the ethanol solvent. The OXD-7 molecules were washed out from the EML during the second spin-coating, leading to the aggregation of the residual PVK molecules in the thin film. The surface roughness of TrOH films decreases gradually from 1.21 nm to 0.70 nm when the content of water increases from 10% to 30%. However, the TrOH film has small grains and aggregates at the amount of water above 30%. As shown in Fig. 7(g) and (h), the significant aggregates can be observed on the surface of the EML, indicating that the EML was not completely covered by TrOH. This can be attributed to the poor contact during spin-coating due to the mismatch between the hydrophobic EML surface and the increased solvent polarity. As shown in Fig. 8, the contact angles between the mixed-solvent (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water) and the EML are gradually increased from 5° to 60° by adding water into ethanol from 0% to 60%. These results indicate that the interfacial morphology is mainly controlled by the wetting characteristics of the EML and TrOH solution. The poorer device performance can be ascribed to the incontinuous TrOH film when the amount of water in TrOH solution is above 40%, which leads to the subdued electron injection from cathode into the EML (Fig. 2).
water) and the EML are gradually increased from 5° to 60° by adding water into ethanol from 0% to 60%. These results indicate that the interfacial morphology is mainly controlled by the wetting characteristics of the EML and TrOH solution. The poorer device performance can be ascribed to the incontinuous TrOH film when the amount of water in TrOH solution is above 40%, which leads to the subdued electron injection from cathode into the EML (Fig. 2).
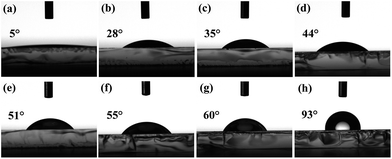 | ||
Fig. 8 Photos of mixed-solvent (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water) contact angles with EML: (a) 10 water) contact angles with EML: (a) 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, (b) 9 0, (b) 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (c) 8 1, (c) 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, (d) 7 2, (d) 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, (e) 6 3, (e) 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, (f) 5 4, (f) 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, (g) 4 5, (g) 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and (h) 0 6 and (h) 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. 10. | ||
Based on above results, the strong dependence of device performance on the solvent composition can be easy to understand. For the device with the TrOH interlayer spin-coated from ethanol, almost all of OXD-7 (about 31 nm, assuming equal densities for all components) was washed out from the EML by ethanol. Holes are the major carriers in the TrOH (ethanol)/Al device. The exciton recombination zone is located close to the interface between the EML and the TrOH interlayer. It has been known that the narrow recombination zone was inferior for the efficiency roll-off due to its high exciton density which induces the triplet exciton quenching process.42 The carrier accumulation at the interface between EML and transport layer is usually responsible for triplet–polaron quenching.41 Therefore, the TrOH (ethanol)/Al device possesses high efficiency roll-off (Fig. 2(c)). Additionally, the TrOH (ethanol)/Al device exhibits higher turn-on voltage than that of the TrOH (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water)/Al device, which can be ascribed to a large barrier for the electron injection from TrOH/Al to PVK. With the increasing water content in TrOH solution, amount of OXD-7 washed out from the EML by ethanol decreases gradually, resulting in efficient electron injection from TrOH/Al to OXD-7. Therefore, the device shows improved efficiency and reduced efficiency roll-off. The turn-on voltages of the devices with TrOH interlayer deposited from mixed-solvent are very close (Table 1). It should be noted that the turn-on voltage of the device increases slightly when the content of water in TrOH solution is above 30%. This can be attributed to the incontinuous TrOH film, which leads to the subdued electron injection from cathode into the EML.
water)/Al device, which can be ascribed to a large barrier for the electron injection from TrOH/Al to PVK. With the increasing water content in TrOH solution, amount of OXD-7 washed out from the EML by ethanol decreases gradually, resulting in efficient electron injection from TrOH/Al to OXD-7. Therefore, the device shows improved efficiency and reduced efficiency roll-off. The turn-on voltages of the devices with TrOH interlayer deposited from mixed-solvent are very close (Table 1). It should be noted that the turn-on voltage of the device increases slightly when the content of water in TrOH solution is above 30%. This can be attributed to the incontinuous TrOH film, which leads to the subdued electron injection from cathode into the EML.
Encouraged by the above results, a single-EML white phosphorescent PLED adopting 10 wt% FIrpic and 0.5 wt% Ir(2-phq)3 co-doped into the PVK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OXD-7 matrix as the EML was fabricated. The TrOH layer was processed from the ethanol/water ratio of 7
OXD-7 matrix as the EML was fabricated. The TrOH layer was processed from the ethanol/water ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The performance of the white PLED is illustrated in Fig. 9. It is shown that the white PLED exhibits a low turn-on voltage of 5.4 V. The device reaches luminance up to 9860 cd m−2 at a voltage of 15.0 V, while it exhibits a peak luminous efficiency of 14.6 cd A−1 at a luminance of 1447 cd m−2. Fig. 9(c) shows the normalized EL spectra of the white PLED at different driving voltages. The EL spectra exhibit two main peaks of 472 and 580 nm, coming from the emission of FIrpic and Ir(2-phq)3, respectively. With the increasing driving voltage, the CIE coordinates shift from (0.372, 0.379) at 7 V to (0.357, 0.375) at 12 V, in which the variation of CIE coordinates is (−0.015, −0.004).
3. The performance of the white PLED is illustrated in Fig. 9. It is shown that the white PLED exhibits a low turn-on voltage of 5.4 V. The device reaches luminance up to 9860 cd m−2 at a voltage of 15.0 V, while it exhibits a peak luminous efficiency of 14.6 cd A−1 at a luminance of 1447 cd m−2. Fig. 9(c) shows the normalized EL spectra of the white PLED at different driving voltages. The EL spectra exhibit two main peaks of 472 and 580 nm, coming from the emission of FIrpic and Ir(2-phq)3, respectively. With the increasing driving voltage, the CIE coordinates shift from (0.372, 0.379) at 7 V to (0.357, 0.375) at 12 V, in which the variation of CIE coordinates is (−0.015, −0.004).
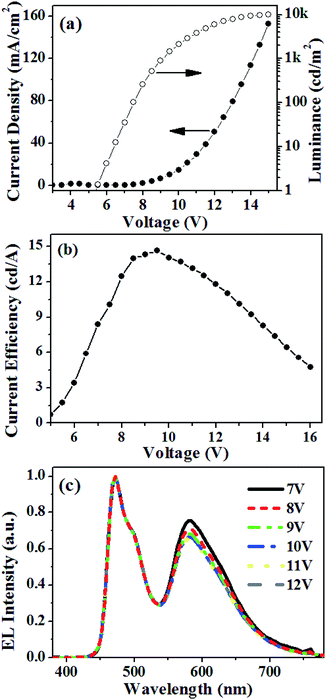 | ||
| Fig. 9 Device characteristics of the solution-processed white phosphorescent OLED: (a) current density–voltage–luminance, (b) efficiency–luminance and (c) EL spectra. | ||
4. Conclusion
In summary, we present highly efficient multilayered PhPLEDs by solution-processing using alcohol/water soluble conjugated starburst macromolecule TrOH as a cathode interlayer. It was found that the performance of the PhPLEDs heavily replied on the solvent composition for TrOH coating. The phosphorescence devices with TrOH interlayer processed from water/ethanol mixed-solvent exhibited a much better performance than that of device with TrOH interlayer processed from ethanol solution. For the devices processed from the ethanol/water ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v) solution, the maximum luminous efficiencies of 23.4 cd A−1 and 14.6 cd A−1 were obtained for green and white PhPLEDs, respectively. We found that the interface erosion of OXD-7 by the ethanol was likely ascribed to the deterioration of the device performance. The erosion of the EML could be greatly suppressed by adding a small amount of water into the ethanol. These results demonstrate that alcohol/water soluble conjugated starburst macromolecule used as the cathode interface material is a promising strategy for applications in fully solution-processed multilayered polymer electronic devices.
3 (v/v) solution, the maximum luminous efficiencies of 23.4 cd A−1 and 14.6 cd A−1 were obtained for green and white PhPLEDs, respectively. We found that the interface erosion of OXD-7 by the ethanol was likely ascribed to the deterioration of the device performance. The erosion of the EML could be greatly suppressed by adding a small amount of water into the ethanol. These results demonstrate that alcohol/water soluble conjugated starburst macromolecule used as the cathode interface material is a promising strategy for applications in fully solution-processed multilayered polymer electronic devices.
Acknowledgements
The project was supported by the National Key Basic Research Program of China (2014CB648300 and 2012CB933301), the NSFC (61204048 and 21422402), the Ministry of Education of China (NCET-13-0872), the Natural Science Foundation of Jiangsu Province (BK20130037, BM2012010 and BK20140060), the Program for Jiangsu Specially-Appointed Professors (RK030STP15001), the Specialized Research Fund for the Doctoral Program of Higher Education (20133223110008, and 20113223110005), the Scientific Research Foundation of NUPT (NY214178 and NY214093), the Jiangsu National Synergetic Innovation Center for Advanced Materials, the Synergetic Innovation Center for Organic Electronics and Information Displays, and the Project Funded by the PAPD of Jiangsu Higher Education Institutions (YX03001).Notes and references
- L. Ying, C.-L. Ho, H. Wu, Y. Cao and W.-Y. Wong, Adv. Mater., 2014, 26, 2459–2473 CrossRef CAS PubMed.
- H. Zheng, Y. N. Zheng, N. L. Liu, N. Ai, Q. Wang, S. Wu, J. H. Zhou, D. G. Hu, S. F. Yu, S. H. Han, W. Xu, C. Luo, Y. H. Meng, Z. X. Jiang, Y. W. Chen, D. Y. Li, F. Huang, J. Wang, J. B. Peng and Y. Cao, Nat. Commun., 2013, 4, 197 Search PubMed.
- S. Hoefle, A. Schienle, M. Bruns, U. Lemmer and A. Colsmann, Adv. Mater., 2014, 26, 2750–2754 CrossRef CAS PubMed.
- V. Cleave, G. Yahioglu, P. Le Barny, R. H. Friend and N. Tessler, Adv. Mater., 1999, 11, 285–288 CrossRef CAS.
- X. Zhang, Z. Wu, B. Jiao, D. Wang, D. Wang, X. Hou and W. Huang, J. Lumin., 2012, 132, 697–701 CrossRef CAS.
- X. Zhang, Q. Hu, J. Lin, Z. Lei, X. Guo, L. Xie, W.-Y. Lai and W. Huang, Appl. Phys. Lett., 2013, 103, 153301 CrossRef.
- X. Zhang, Z. Lei, Q. Hui, J. Lin, Y. Chen, L. Xie, W.-Y. Lai and W. Huang, Appl. Phys. Express, 2014, 7, 101601 CrossRef.
- A. M. Douvas, M. Vasilopoulou, D. G. Georgiadou, A. Soultati, D. Davazoglou, N. Vourdas, K. P. Giannakopoulos, A. G. Kontos, S. Kennou and P. Argitis, J. Mater. Chem. C, 2014, 2, 6290–6300 RSC.
- J. Lee, H. Youn and M. Yang, Org. Electron., 2015, 22, 81–85 CrossRef CAS.
- H. Lee, Y. Kwon and C. Lee, J. Soc. Inf. Disp., 2012, 20, 640–645 CrossRef CAS.
- F. Jiang, W. C. H. Choy, X. Li, D. Zhang and J. Cheng, Adv. Mater., 2015, 27, 2930–2937 CrossRef CAS PubMed.
- B. R. Lee, J. W. Kim, D. Kang, D. W. Lee, S. J. Ko, H. J. Lee, C. L. Lee, J. Y. Kim, H. S. Shin and M. H. Song, ACS Nano, 2012, 6, 2984–2991 CrossRef CAS PubMed.
- G. Liaptsis and K. Meerholz, Adv. Funct. Mater., 2013, 23, 359–365 CrossRef CAS.
- W. Jiang, X. Ban, M. Ye, Y. Sun, L. Duan and Y. Qiu, J. Mater. Chem. C, 2015, 3, 243–246 RSC.
- J. Park, C. Lee, J. Jung, H. Kang, K.-H. Kim, B. Ma and B. J. Kim, Adv. Funct. Mater., 2014, 24, 7588–7596 CrossRef CAS.
- R. H. Friend, R. W. Gymer, A. B. Holmes, J. H. Burroughes, R. N. Marks, C. Taliani, D. D. C. Bradley, D. A. Dos Santos, J. L. Bredas, M. Logdlund and W. R. Salaneck, Nature, 1999, 397, 121–128 CrossRef CAS.
- Y. H. Niu, M. S. Liu, J. W. Ka, J. Bardeker, M. T. Zin, R. Schofield, Y. Chi and A. K. Y. Jen, Adv. Mater., 2007, 19, 300–304 CrossRef CAS.
- Q. Zhao, W. Zhang, Z. Fan, J. Li, X. Chen, G. Luo and X. Zhang, Synth. Met., 2015, 204, 70–75 CrossRef CAS.
- Y. D. Zhang, C. Zuniga, S. J. Kim, D. K. Cai, S. Barlow, S. Salman, V. Coropceanu, J. L. Bredas, B. Kippelen and S. Marder, Chem. Mater., 2011, 23, 4002–4015 CrossRef CAS.
- M. Cai, T. Xiao, E. Hellerich, Y. Chen, R. Shinar and J. Shinar, Adv. Mater., 2011, 23, 3590–3596 CrossRef CAS PubMed.
- Z. C. Ding, R. B. Xing, Q. A. Fu, D. G. Ma and Y. C. Han, Org. Electron., 2011, 12, 703–709 CrossRef CAS.
- D. A. Pardo, G. E. Jabbour and N. Peyghambarian, Adv. Mater., 2000, 12, 1249–1252 CrossRef CAS.
- C. M. Zhong, C. H. Duan, F. Huang, H. B. Wu and Y. Cao, Chem. Mater., 2011, 23, 326–340 CrossRef CAS.
- K. Morii, M. Ishida, T. Takashima, T. Shimoda, Q. Wang, M. K. Nazeeruddin and M. Gratzel, Appl. Phys. Lett., 2006, 89, 183510 CrossRef.
- H. J. Bolink, E. Coronado, D. Repetto, M. Sessolo, E. M. Barea, J. Bisquert, G. Garcia-Belmonte, J. Prochazka and L. Kavan, Adv. Funct. Mater., 2008, 18, 145–150 CrossRef CAS.
- M. T. Greiner, L. Chai, M. G. Helander, W. M. Tang and Z. H. Lu, Adv. Funct. Mater., 2012, 22, 4557–4568 CrossRef CAS.
- H. Choi, J. S. Park, E. Jeong, G. H. Kim, B. R. Lee, S. O. Kim, M. H. Song, H. Y. Woo and J. Y. Kim, Adv. Mater., 2011, 23, 2759–2763 CrossRef CAS PubMed.
- M. Sessolo and H. J. Bolink, Adv. Mater., 2011, 23, 1829–1845 CrossRef CAS PubMed.
- F. Huang, L. T. Hou, H. B. Wu, X. H. Wang, H. L. Shen, W. Cao, W. Yang and Y. Cao, J. Am. Chem. Soc., 2004, 126, 9845–9853 CrossRef CAS PubMed.
- W. L. Ma, P. K. Iyer, X. Gong, B. Liu, D. Moses, G. C. Bazan and A. J. Heeger, Adv. Mater., 2005, 17, 274–277 CrossRef CAS.
- C. Hoven, R. Yang, A. Garcia, A. J. Heeger, T. Q. Nguyen and G. C. Bazan, J. Am. Chem. Soc., 2007, 129, 10976–10977 CrossRef CAS PubMed.
- H. B. Wu, F. Huang, Y. Q. Mo, W. Yang, D. L. Wang, J. B. Peng and Y. Cao, Adv. Mater., 2004, 16, 1826–1830 CrossRef CAS.
- H. B. Wu, F. Huang, Y. Q. Mo, W. Yang, D. L. Wang, J. B. Peng and Y. Cao, Adv. Mater., 2004, 16, 1826–1830 CrossRef CAS.
- W.-D. Xu, X.-W. Zhang, Q. Hu, L. Zhao, X. Y. Teng, W.-Y. Lai, R. D. Xia, J. Nelson, W. Huang and D. D. C. Bradley, Org. Electron., 2014, 15, 1244–1253 CrossRef CAS.
- Y. Xu, R. Yang, J. Peng, A. A. Mikhailovsky, Y. Cao, T.-Q. Nguyen and G. C. Bazan, Adv. Mater., 2009, 21, 584–588 CrossRef CAS PubMed.
- D. An, J. H. Zou, H. B. Wu, J. B. Peng, W. Yang and Y. Cao, Org. Electron., 2009, 10, 299–304 CrossRef CAS.
- F. Huang, Y. Zhang, M. S. Liu and A. K. Y. Jen, Adv. Funct. Mater., 2009, 19, 2457–2466 CrossRef CAS.
- J. H. Seo, R. Q. Yang, J. Z. Brzezinski, B. Walker, G. C. Bazan and T. Q. Nguyen, Adv. Mater., 2009, 21, 1006–1011 CrossRef CAS.
- W.-D. Xu, W.-Y. Lai, Q. Hu, X.-Y. Teng, X.-W. Zhang and W. Huang, Polym. Chem., 2014, 5, 2942–2950 RSC.
- Y. Chen, Z. Lei, X. Zhang, S. Chu, W. Xu, B. Liu, C. Ou, L. Xie, Q. Fan, W.-Y. Lai and W. Huang, J. Lumin., 2016, 170, 50–55 CrossRef CAS.
- C. Murawski, K. Leo and M. C. Gather, Adv. Mater., 2013, 25, 6801–6827 CrossRef CAS PubMed.
- N. C. Giebink and S. R. Forrest, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 235215 CrossRef.
- C.-C. Chen, B.-C. Huang, M.-S. Lin, Y.-J. Lu, T.-Y. Cho, C.-H. Chang, K.-C. Tien, S.-H. Liu, T.-H. Ke and C.-C. Wu, Org. Electron., 2010, 11, 1901–1908 CrossRef CAS.
- I. W. Wu, P.-S. Wang, W.-H. Tseng, J.-H. Chang and C.-I. Wu, Org. Electron., 2012, 13, 13–17 CrossRef CAS.
- B. Park, O. E. Kwon, S. H. Yun, H. G. Jeon and Y. H. Huh, J. Mater. Chem. C, 2014, 2, 8614–8621 RSC.
- P. D'Angelo, M. Barra, A. CassineSe, M. G. Maglione, P. Vacca, C. Minarini and A. Rubino, Solid-State Electron., 2007, 51, 123–129 CrossRef.
- Y. Zhang, F. Huang, Y. Chi and A. K. Y. Jen, Adv. Mater., 2008, 20, 1565–1570 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |