The metamorphosis of vascular stents: passive structures to smart devices
Abstract
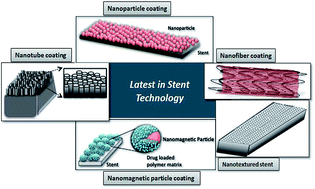
Drug-eluting stents (DES) represent a major milestone in stent technology, saving the lives of millions worldwide afflicted with cardiovascular disease. Though a wide range of therapeutic approaches has been employed for treatment of coronary artery diseases, stent deployment has become the most extensively practiced method to open blocked arteries in comparison with surgical revascularisation. In recent years, the bare metal stent has evolved into more effective drug-eluting systems and the encouraging results from initial clinical trials have instilled hope among cardiologists to reduce the number of fatalities caused by cardiovascular diseases through the deployment of drug-eluting stents. Over the years, the stents have evolved from a passive framework that maintained blood flow through the blood vessels into multi-functional devices that could also serve to mitigate conditions leading to the blockage of the blood vessels while promoting re-endothelialization of the blood vessel. This review traces the evolution of the stents from the first generation structures to the modern versions that overcome their limitations. A glimpse into the exciting future of stent technology is also provided.

 Please wait while we load your content...
Please wait while we load your content...