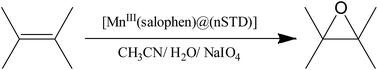Manganese(III)salophen supported on a silica containing triazine dendrimer: an efficient catalyst for epoxidation of alkenes with sodium periodate†
Mahsa Asadniaye Fardjahromi,
Majid Moghadam*,
Shahram Tangestaninejad*,
Valiollah Mirkhani and
Iraj Mohammadpoor-Baltork
Department of Chemistry, Catalysis Division, University of Isfahan, Isfahan, 81746-73441, Iran. E-mail: moghadamm@sci.ui.ac.ir; stanges@sci.ui.ac.ir; Fax: +98-31-36689732; Tel: +98-31-37934920
First published on 1st February 2016
Abstract
In this paper, Mn(III) salophen supported on a nanosilica triazine dendrimer was synthesized and characterized by FT-IR, UV-vis spectroscopic methods, SEM, TEM, TGA and ICP analyses. This catalyst was used in the epoxidation of different cyclic and linear alkenes with sodium periodate under agitation with magnetic stirring. Furthermore, the influence of vital reaction parameters such as solvent, oxidant and amount of catalyst on the epoxidation of alkenes was investigated. This heterogeneous catalyst shows high stability and reusability in the epoxidation of alkenes.
Introduction
Epoxidation of alkenes has recently gained increasing attention in organic synthesis since epoxides can transform into mono- or bi-functional organic products such as amino alcohols, allylic alcohols, ketones, polyethers and etc.1–3Schiff base complexes of Mn, Fe, Ru, Cr, Co, Mo and V have found many applications in organic chemistry. These compounds have been used as catalysts in the oxidation of alkenes and alkanes,4–16 sulfides,17–19 amines,20,21 alcohols,22,23 Diels–Alder reactions,24 polymerization reactions and electro-reduction.25,26 Among different metal Schiff base complexes, manganese Schiff base complexes are more suitable for epoxidation of olefins since they are attractive redox active systems and demonstrate a high catalytic activity and selectivity.27,28
Since the pioneering synthesis of chiral manganese(II)salen complex by Jacobsen group,27 a wide variety of manganese Schiff base complexes with different activity and selectivity were reported.25,29,30 Although these homogenous catalysts showed high activity in the epoxidation of olefins, they often suffer from difficulty in recovery and reusability.25 To overcome these problems, considerable efforts have been devoted to immobilize these complexes on various supports such as zeolites,8,31,32 silica,33 organic polymers,34 clays,5,35 magnetic nanoparticles,36,37 ion-exchange resins38 and carbon nanotubes.15,39 However, the catalytic activity of some of these heterogeneous catalysts decreases upon immobilization due to the reduction in accessibility of active sites.
Dendrimers are spatial type of polymers with highly branched and tree-like molecular structures which their synthesis and properties have attracted much attention in catalysis and pharmaceutical applications.40–44 In addition, due to the abundant of functional groups at the surface of dendrimers, they can be easily bonded with different metal complexes and act as suitable hosts for homogenous catalysts.45 Recently, triazine based dendrimers have received considerable attention due to cost, chemoselective reactivity of the starting material and easy synthesis.46–50
Immobilization of metal-containing dendrimer catalysts on nanoparticles facilitates their recovery and reuse.51,52
In this work, we focus our efforts on the synthesis of Mn(III) salophen supported on nanosilica triazine dendrimer, [MnIII(salophen)Cl@nSTD], as a new nanocatalyst for epoxidation of different alkenes with NaIO4 (Scheme 1).
Experimental
Materials and reagents
All chemicals were purchased from Merck and Aldrich chemical companies. Mn salophen was prepared according to the literature procedure.53 The nanosilica triazine dendrimer was prepared according to our previously reported work.54Physico-chemical measurements
FT-IR spectra were recorded on a Jasco 6300D instrument. Diffuse reflectance UV-vis (DR UV-vis) spectra were obtained on a JASCO V-670 spectrophotometer. Elemental analyses were performed on a LECO, CHNS-932 analyzer. Thermogravimetric analyses (TGA) were carried out on a Mettler TG50 instrument under oxygen flow at a uniform heating rate of 20 °C min−1 in the range of 300–600 °C. Gas chromatography (GC) experiments were performed with a Shimadzu GC-16A instrument equipped with a FID detector using a 2 m column packed with silicon DC-200 or Carbowax 20m. The N2 (flow rate 80 ml min−1), air (flow rate 90 ml min−1) and H2 were used as carrier, oxidizing and fuel gases, respectively. The yields were determined using standard addition method and in this manner, n-decane was used as internal standard. The oven and column temperatures and also the retention times for reactants and products are provided in the ESI (Table S1†). The scanning electron micrographs were recorded on a Hitachi S-4700 field emission scanning electron microscope (FE-SEM). The samples were coated with gold for preparing of SEM-EDX images. The transmission electron microscopy (TEM) was carried out on a Philips CM10 Transmission Electron Microscope operating at 100 kV. The Mn content of the catalyst was determined by a Jarrell-Ash 1100 ICP analyses.Preparation of triazine dendrimer-supported manganese salophen catalyst, [Mn(salophen)Cl@nSTD]
To a solution of Mn(salophen)Cl (0.2 g) in CH3CN (50 ml), nSTD (1 g) was added and the mixture was refluxed for 48 h. The resulting bright brown solid was filtered, washed thoroughly with MeOH and CH3CN. The unreacted Mn(salophen)Cl was removed by extraction with CH3CN by a Soxhlet apparatus. The catalyst was dried under vacuum for several hours.General procedure for epoxidation of alkenes with sodium periodate catalysed by [Mn(salophen)Cl@nSTD]
In a round-bottom flask equipped with a magnetic stirrer bar, a solution of NaIO4 (1 mmol) in H2O (2.5 ml) was added to a solution of alkene (0.5 mmol) in acetonitrile (2.5 ml). Afterward, [Mn(salophen)Cl@nSTD] (150 mg, 0.02 mmol) was added to the solution and the mixture was stirred at room temperature under air atmosphere. The progress of the reaction was monitored by GC. At the end of the reaction, the catalyst was removed by simple filtration and washed with the adequate amount of acetonitrile and H2O. Later on, the products were extracted with Et2O and purified on a silica gel column.Results and discussion
Characterization of catalyst [Mn(salophen)Cl@nSTD]
The preparation route for catalyst is shown in Scheme 2. The first indication for attachment of Mn(salophen)Cl to nSTD is changing the dendrimer color from white to bright brown.This new catalyst was characterized by FT-IR, UV-vis spectroscopies, TEM, SEM and TGA. The amount of manganese supported on the dendrimer was measured by ICP which showed a value of about 0.134 mmol per gram of the heterogeneous catalyst.
The FT-IR spectra of nSTD (G2) and [Mn(salophen)Cl@nSTD] are shown in Fig. S1.† Due to the masking of Mn Schiff base C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond with those of cyanuric chloride, no further information was obtained.
N bond with those of cyanuric chloride, no further information was obtained.
The UV-vis spectrum provided an informative evidence for immobilization of the [Mn(salophen)Cl] on triazine dendrimer. The absorption spectrum of triazine dendrimer shows no peak above 400 nm. However, the [Mn(salophen)Cl@nSTD] shows a peak at 420 nm which is attributed to the ligand-to-metal charge transfer of [Mn(salophen)Cl] (Fig. 1).55,56
The surface morphology and the size of [Mn(salophen)Cl@nSTD] and nSTD particles were studied by field emission electron microscopy. As can be seen in Fig. 3a and b, the particles were agglomerated and have a semi-spherical shape. It is clearly observed that the attachment of [Mn(salophen)Cl] to nSTD was led to breaking the agglomerates and reduction of particles size. The presence of manganese in the energy dispersive X-ray spectrum of [Mn(salophen)Cl@nSTD] is another evidence for immobilization of Mn(salophen)Cl on nSTD (Fig. 2c). The SEM-EDX spectroscopy also shows that Si, O, C, Cl are present in the texture of the [Mn(salophen)Cl@nSTD]. The Cl peak is attributed to the chloride in the Mn(salophen)Cl. The Au peak is corresponded to coating the sample with gold during the sample preparation for FE-SEM analysis.
 | ||
| Fig. 2 FE-SEM image of: (a) nSTD; (b) [Mn(salophen)Cl@nSTD] and (c) SEM-EDX spectrum of [Mn(salophen)Cl@nSTD]. | ||
The high field transmission electron micrograph of [Mn(salophen)Cl@nSTD] is shown in Fig. 3. The dark regions in TEM image correspond to manganese species and the colorless regions show the nanosilica triazine dendrimer.
Further characterization of [Mn(salophen)Cl@nSTD] was performed by thermogravimetric analyses. The weight loss of nSTD and catalyst as a function of temperature in the range of 300 to 600 °C was measured. The thermograms showed two thermal decomposition steps (Fig. 4). The organic weight loss of nSTD and the supported catalyst were 66% and 73%, respectively. This indicates that the amount of organic material increased upon attachment of manganese salophen to nSTD.
Catalytic experiments
The catalytic activity of the prepared catalyst was studied in the epoxidation of different olefins with NaIO4 at room temperature. It is clear that the type of solvent and oxidant, and amount of catalyst are crucial parameters for obtaining the higher catalytic activity.First, different quantities of [Mn(salophen)Cl@nSTD] were used in the epoxidation of cyclooctene with NaIO4. The results indicated that 150 mg (0.02 mmol) of the catalyst is the best amount for epoxidation of 0.5 mmol of cyclooctene (Table 1). The nature of oxygen donor is another essential factor that needs to be optimized carefully. For this purpose, the effect of various oxidants such as NaOCl, NaIO4, H2O2, tert-butyl hydroperoxide was investigated in the epoxidation of cyclooctene catalyzed by [Mn(salophen)Cl@nSTD]. The obtained results are summarized in Table 2. When TBHP and NaOCl were used as oxidant, the epoxidation yields were 5% and 24% respectively while the yield was 64% using H2O2 as oxygen donor. Among the different oxidant used, sodium periodate was selected as the oxygen source due to the following reasons (i) it can give highest oxidation conversion; (ii) it is inert in the absence of catalyst, and (iii) it is highly soluble in CH3CN/H2O mixture.
The effect of the reaction medium on the epoxidation of cyclooctene was also investigated. Aqueous mixtures of methanol, ethanol, acetone and acetonitrile (single phase systems), dichloromethane and carbon tetrachloride (two phase system with n-Bu4NBr as phase transfer catalyst) were checked in the epoxidation of cyclooctene with NaIO4. The data in Table 3 show that the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of acetonitrile
1 mixture of acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water was the most appropriate reaction medium due to the higher epoxide yield. The higher catalytic activity in aqueous acetonitrile is attributed to the polarity of solvent and also high solubility of periodate in CH3CN/H2O mixture.
water was the most appropriate reaction medium due to the higher epoxide yield. The higher catalytic activity in aqueous acetonitrile is attributed to the polarity of solvent and also high solubility of periodate in CH3CN/H2O mixture.
| Entry | Solvent | Epoxide yieldb (%) after 2 h |
|---|---|---|
| a Reaction conditions: cyclooctene (0.5 mmol), NaIO4 (1 mmol), catalyst (150 mg, 0.02 mmol Mn), CH3CN/H2O (5 ml/2.5 ml).b GC yield based on the starting cyclooctene. | ||
| 1 | CH3CN/H2O | 95 |
| 2 | CHCl3/H2O | 14 |
| 3 | CH2Cl2/H2O | 38 |
| 4 | CH3COCH3/H2O | 68 |
| 5 | CH3CH2OH/H2O | 43 |
| 6 | CH3OH/H2O | 58 |
Under the optimized reaction conditions, epoxidation of different alkenes with NaIO4 was performed at room temperature in the presence of [Mn(salophen)Cl@nSTD] in CH3CN/H2O. Our experimental results showed that the proposed catalyst is able to oxidize several alkenes to their corresponding epoxides in good to excellent yield (Table 4).
| Entry | Alkene | Time (h) | Conversionb,c (%) | Selectivity (%) | TOF (h−1) | |
|---|---|---|---|---|---|---|
| a Reaction conditions: alkene (0.5 mmol), NaIO4 (1 mmol), catalyst (150 mg, 0.02 mmol), CH3CN/H2O (5 ml/2.5 ml).b GC yield based on starting alkene.c Yield in the parenthesis refers to epoxide yield.d By-product is benzaldehyde.e By-product is acetophenone. | ||||||
| 1 | 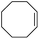 |
 |
2.5 | 95 (95) | 100 | 9.5 |
| 2 | 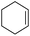 |
 |
3 | 90 (90) | 100 | 7.5 |
| 3 | 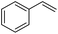 |
 |
3 | 83 (74)d | 89 | 6.91 |
| 4 | 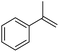 |
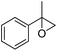 |
3 | 88 (72)e | 81 | 7.33 |
| 5 | 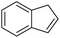 |
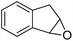 |
3 | 67 (67) | 100 | 5.58 |
| 6 |  |
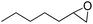 |
3.5 | 71 (71) | 100 | 5.07 |
| 7 |  |
 |
3.5 | 64 (64) | 100 | 4.57 |
| 8 |  |
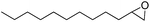 |
3.5 | 52 (52) | 100 | 3.71 |
Linear, cyclic and phenyl-substituted alkenes were used as substrates in this system. Electron-rich cyclic olefins are more reactive than the electron-poor terminal ones which can reflect the electrophilic nature of oxygen transfer from manganese-oxo intermediate to the olefinic double bond.
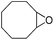
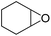
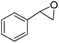
Cyclooctene and cyclohexene were oxidized in high yield and 100% selectivity (entries 1 and 2). Styrene and α-methylstyrene were epoxidized in 90% and 74% yield respectively, and only trace amount of benzaldehyde and acetophenone were produced as minor products (entries 3 and 4). These related carbonyl compounds were achieved as a by-products resulting from ring-opening reaction of the corresponding epoxides. Indene transformed into its corresponding epoxide with 100% selectivity and 67% yield. Epoxidation of linear alkenes such as 1-heptene, 1-octene and 1-dodecene was achieved and the corresponding epoxides obtained in good yields and 100% selectivity (entries 6, 7 and 8).
In biomimetic systems, it is necessary to add an axial base to increase the catalytic activity of these catalysts. One of the most remarkable advantages of [Mn(salophen)Cl@nSTD] catalytic system is that there is no need to add axial ligand. The amine groups not only act as support but also play the role of axial ligand. In addition, the covalent bonding between the amine groups at the surface of triazine dendrimer and the manganese salophen prevents the formation of inactive dimeric μ-oxo manganese(IV) species. On the other hand such immobilization isolate the catalytic active sites and facilities the access of reactant to the catalyst.
The results obtained by [Mn(salophen)Cl@nSTD]/NaIO4 catalytic system were compared with some of those reported in the literature (Table 5). As can be seen, the catalytic systems, MoOx inserted within MCM-41 and [Mn(salen)(H2O)]PF6 supported on clay, gave the higher turnover frequency (TOF). However, the TBHP is highly reactive, flammable and toxic oxidant compared with NaIO4 which is used as a mild oxidant in the present work. Compared to other catalytic systems, the present method is more reactive in terms of catalyst amount, reaction time and/or the epoxide yield. The higher catalytic activity of this catalytic system can be attributed to the dispersion of catalyst on the nanosilica triazine dendromer which isolate the catalytic active sites.
| Row | Catalyst | Catalyst amount (mmol) | Time (h) | Yield (%) | TOF (h−1) | Oxidant | Cyclooctene (mmol) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Manganese(III) salophen supported on silica containing triazine dendrimer | 0.02 | 2.5 | 95 | 9.5 | NaIO4 | 0.5 | This work |
| 2 | Zeolite-encapsulated Ru(III) salophen | 0.01 | 5 | 100 | 10.0 | NaIO4 | 0.5 | 8 |
| 3 | Mn[(salen)(H2O)]PF6 supported on clay | 0.011 | 0.75 | 26 | 26.67 | TBHP | 0.6 | 5 |
| 4 | Polymer-supported bis(2-hydroxyanyl) acetylacetonatomolybdenyl Schiff base | 0.029 | 1.5 | 97 | 11.14 | TBHP | 0.5 | 6 |
| 5 | Zeolite-encapsulated Mn(III)-salophen | 0.45 | 10 | 92 | 2.1 | NaIO4 | 0.5 | 31 |
| 6 | Mn(III) salophen supported on imidazole modified silica | 0.042 | 3 | 95 | 7.38 | NaIO4 | 0.5 | 33 |
| 7 | Mn(III) salophen supported on polystyrene-bound imidazole | 0.042 | 2.5 | 98 | 4.67 | NaIO4 | 0.5 | 34 |
| 8 | Mn(III) salophen supported on 1,4-diaminobenzene modified MWCNTs | 0.06 | 2.5 | 99 | 6.60 | NaIO4 | 1 | 39 |
| 9 | Mn(III) salophen supported on polystyrene-bound 1,4-phenylenediamine | 0.054 | 2.5 | 98 | 3.62 | NaIO4 | 0.5 | 57 |
| 10 | Manganese(III)tetraphenylporphyrin supported on 2-aminothiazole modified MWCNTs | 0.018 | 3 | 92 | 8.29 | NaIO4 | 0.5 | 58 |
| 11 | Manganese(III)tetraphenylporphyrin supported on 4-aminopyridine modified MWCNTs | 0.02 | 3 | 94 | 7.56 | NaIO4 | 0.5 | 59 |
| 12 | Achiral molybdenum(VI) dioxo complex supported on MCM-41 | 0.0087 | 24 | 28 | 1.07 | TBHP | 8 | 60 |
| 13 | Chiral molybdenum(VI) dioxo complex supported on MCM-41 | 0.0087 | 24 | 30 | 1.14 | TBHP | 8 | 60 |
| 14 | MoOx inserted within mesoporous silica (MCM-41, SBA-15) and grafted onto silica | 2.254 | 3 | 97 | 21.56 | TBHP | 1.5 | 61 |
| 15 | Dioxomolybdenum(VI) complex supported on MCM-41 functionalized with pyrazolyl pyridine | 0.145 | 8 | 100 | 6.20 | TBHP | 7.2 | 62 |
Catalyst reuse and stability
Another prominent feature of [Mn(salophen)Cl@nSTD] catalyst is its simple recovery and good reusability in the epoxidation reactions. The epoxidation of cyclooctene was chosen as a model reaction. At the end of each run, the catalyst was separated by simple filtration and washed with acetonitrile and H2O and dried at room temperature before using it in the subsequent runs (Fig. 5).The results showed the epoxide yields after first two runs were 95% and 91% and the amount of the manganese leached, determined by ICP by analyzing the filtrates after each run, were 5% and 3%, respectively. The catalyst reusability was continued for four extra runs. After sixth run, the epoxide yield was 82%. Analysis of the filtrates showed no Mn leaching in these runs. On the other hand, the amount of Mn in the reused catalyst after sixth run showed a value of about 0.125 mmol g−1.
Conclusion
In this paper, the [MnIII(salophen)Cl] immobilized on nanosilica triazine dendrimer was successfully synthesized and characterized. The [MnIII(salophen)Cl@nSTD] catalyst was found as an efficient catalyst in the epoxidation of alkenes under mild conditions with high yield and excellent selectivity. Moreover, this catalyst can be reused four runs without significant loss of its initial activity. This high catalyst reusability and recovery can be referred to the strong covalent bond between manganese salophen and nanosilica triazine dendrimer.Acknowledgements
Partial support of this work by the research council of the University of Isfahan is acknowledged.References
- J. A. Birrell and E. N. Jacobsen, Org. Lett., 2013, 15, 2895–2897 CrossRef CAS PubMed.
- Z. Wang, Y.-T. Cui, Z.-B. Xu and J. Qu, J. Org. Chem., 2008, 73, 2270–2274 CrossRef CAS PubMed.
- A. J. McGrath, W. Shi, C. G. Rodriguez, E. J. Kramer, C. J. Hawker and N. A. Lynd, Polym. Chem., 2015, 6, 1465–1473 RSC.
- X. Wang, S. Wu, Z. Li, X. Yang, H. Su, J. Hu, Q. Huo, J. Guan and Q. Kan, Microporous Mesoporous Mater., 2016, 221, 58–66 CrossRef CAS.
- P. S. Dixit and K. Srinivasan, Inorg. Chem., 1988, 27, 4507–4509 CrossRef CAS.
- G. Grivani and A. Akherati, Inorg. Chem. Commun., 2013, 28, 90–93 CrossRef CAS.
- J. Zhang, P. Jiang, Y. Shen, W. Zhang and X. Li, Microporous Mesoporous Mater., 2015, 206, 161–169 CrossRef CAS.
- M. Moosavifar, S. Tangestaninejad, M. Moghadam, V. Mirkhani and I. Mohammadpoor-Baltork, J. Mol. Catal. A: Chem., 2013, 377, 92–101 CrossRef CAS.
- G. Xu, Q.-H. Xia, X.-H. Lu and H.-J. Zhan, J. Mol. Catal. A: Chem., 2007, 266, 180–187 CrossRef CAS.
- B. Qi, X.-H. Lu, S.-Y. Fang, J. Lei, Y.-L. Dong, D. Zhou and Q.-H. Xia, Catal. Commun., 2011, 334, 44–51 CAS.
- X.-L. Wei, X.-H. Lu, X.-T. Ma, C. Peng, H.-Z. Jiang, D. Zhou and Q.-H. Xia, Catal. Commun., 2015, 61, 48–52 CrossRef CAS.
- X.-H. Lu, J. Lei, X.-L. Wei, X.-T. Ma, D. Zhou and Q.-H. Xia, J. Mol. Catal. A: Chem., 2015, 400, 71–80 CrossRef CAS.
- X.-H. Lu, Q.-H. Xia, H.-J. Zhan, H.-X. Yuan, C.-P. Ye, K.-X. Su and G. Xu, J. Mol. Catal. A: Chem., 2006, 250, 62–69 CrossRef CAS.
- D. Saha, T. Maity, R. Bera and S. Koner, Polyhedron, 2013, 56, 230–236 CrossRef CAS.
- A. Mavrogiorgou, M. Papastergiou, Y. Deligiannakis and M. Louloudi, J. Mol. Catal. A: Chem., 2014, 393, 8–17 CrossRef CAS.
- L. Ma, F. Su, X. Zhang, D. Song, Y. Guo and J. Hu, Microporous Mesoporous Mater., 2014, 184, 37–46 CrossRef CAS.
- H. Mahdavi, M. Nikoorazm, A. Ghorbani-Choghamarani and S. Arshadi, J. Porous Mater., 2015, 22, 1–8 CrossRef CAS.
- M. Bagherzadeh and M. Amini, Inorg. Chem. Commun., 2009, 12, 21–25 CrossRef CAS.
- A. Jabbari, H. Mahdavi, M. Nikoorazm and A. Ghorbani-Choghamarani, J. Porous Mater., 2015, 22, 1111–1118 CrossRef CAS.
- V. Mirkhani, S. Tangestaninejad, M. Moghadam and M. Moghbel, Bioorg. Med. Chem., 2004, 12, 4673–4677 CrossRef CAS PubMed.
- P. K. Khatri, S. Choudhary, R. Singh, S. L. Jain and O. P. Khatri, Dalton Trans., 2014, 43, 8054–8061 RSC.
- H. P. Mungse, S. Verma, N. Kumar, B. Sain and O. P. Khatri, J. Mater. Chem., 2012, 22, 5427–5433 RSC.
- C. Parmeggiani and F. Cardona, Green Chem., 2012, 14, 547–564 RSC.
- E. R. Jarvo, B. M. Lawrence and E. N. Jacobsen, Angew. Chem., Int. Ed., 2005, 44, 6043–6046 CrossRef CAS PubMed.
- K. Gupta and A. K. Sutar, Coord. Chem. Rev., 2008, 252, 1420–1450 CrossRef CAS.
- Y.-D. Zhao, D.-W. Pang, Z.-L. Wang, J.-K. Cheng, Z.-F. Luo, C.-J. Feng, H.-Y. Shen and X.-C. Zhang, Acta Chim. Sin., 1998, 56, 178–183 CAS.
- W. Zhang, J. L. Loebach, S. R. Wilson and E. N. Jacobsen, J. Am. Chem. Soc., 1990, 112, 2801–2803 CrossRef CAS.
- C. P. Horwitz, S. E. Creager and R. W. Murray, Inorg. Chem., 1990, 29, 1006–1011 CrossRef CAS.
- R. Azadbakht, A. A. Manesh, M. Malayeri and B. Dehghani, New J. Chem., 2015, 39, 6459–6464 RSC.
- P. Suresh, S. Srimurugan, R. T. Dere, R. V. Ragavan and V. S. Gopinath, Tetrahedron: Asymmetry, 2013, 24, 669–676 CrossRef CAS.
- V. Mirkhani, M. Moghadam, S. Tangestaninejad, B. Bahramian and A. Mallekpoor-Shalamzari, Appl. Catal., A, 2007, 321, 49–57 CrossRef CAS.
- B. Qi, X.-H. Lu, D. Zhou, Q.-H. Xia, Z.-R. Tang, S.-Y. Fang, T. Pang and Y.-L. Dong, J. Mol. Catal. A: Chem., 2010, 322, 73–79 CrossRef CAS.
- V. Mirkhani, M. Moghadam, S. Tangestaninejad and B. Bahramian, Appl. Catal., A, 2006, 313, 122–129 CrossRef CAS.
- V. Mirkhani, M. Moghadam, S. Tangestaninejad and B. Bahramian, Appl. Catal., A, 2006, 311, 43–50 CrossRef CAS.
- I. Kuźniarska-Biernacka, C. Pereira, A. P. Carvalho, J. Pires and C. Freire, Appl. Clay Sci., 2011, 53, 195–203 CrossRef.
- M. Torki, S. Tangestaninejad, V. Mirkhani, M. Moghadam, I. Mohammadpoor-Baltork and A. R. Khosropour, J. Inorg. Organomet. Polym. Mater., 2013, 23, 923–929 CrossRef CAS.
- M. Esmaeilpour, J. Javidi, F. N. Dodeji and M. M. Abarghoui, Transition Met. Chem., 2014, 39, 797–809 CrossRef CAS.
- V. Mirkhani, M. Moghadam, S. Tangestaninejad and B. Bahramian, Monatsh. Chem., 2007, 138, 1303–1308 CrossRef CAS.
- S. Tangestaninejad, M. Moghadam, V. Mirkhani, I. Mohammadpoor-Baltork and M. S. Saeedi, Appl. Catal., A, 2010, 381, 233–241 CrossRef CAS.
- H. Yang and W. J. Kao, J. Biomater. Sci., Polym. Ed., 2006, 17, 3–19 CrossRef CAS PubMed.
- S. Svenson and D. A. Tomalia, Adv. Drug Delivery Rev., 2005, 57, 2106–2129 CrossRef CAS PubMed.
- A. S. King and L. J. Twyman, J. Chem. Soc., Perkin Trans. 1, 2002, 2209–2218 RSC.
- V. Chechik and R. M. Crooks, J. Am. Chem. Soc., 2000, 122, 1243–1244 CrossRef CAS.
- D. Astruc and F. Chardac, Chem. Rev., 2001, 101, 2991–3024 CrossRef CAS PubMed.
- J. N. Reek, S. Arévalo, R. van Heerbeek, P. C. Kamer and P. W. Van Leeuwen, Adv. Catal., 2006, 49, 71–151 CAS.
- W. Zhang and E. E. Simanek, Org. Lett., 2000, 2, 843–845 CrossRef CAS PubMed.
- S. Lalwani, V. J. Venditto, A. Chouai, G. E. Rivera, S. Shaunak and E. E. Simanek, Macromolecules, 2009, 42, 3152–3161 CrossRef CAS PubMed.
- J. Lim, M. A. Mintzer, L. M. Perez and E. E. Simanek, Org. Lett., 2010, 12, 1148–1151 CrossRef CAS PubMed.
- O. M. Merkel, M. A. Mintzer, J. Sitterberg, U. Bakowsky, E. E. Simanek and T. Kissel, Bioconjugate Chem., 2009, 20, 1799–1806 CrossRef CAS PubMed.
- E. Hollink, S. E. Tichy and E. E. Simanek, Ind. Eng. Chem. Res., 2005, 44, 1634–1639 CrossRef CAS.
- S. Antebi, P. Arya, L. E. Manzer and H. Alper, J. Org. Chem., 2002, 67, 6623–6631 CrossRef CAS PubMed.
- P. P. Zweni and H. Alper, Adv. Synth. Catal., 2004, 346, 849–854 CrossRef CAS.
- D. Chen and A. Martell, Inorg. Chem., 1987, 26, 1026–1030 CrossRef CAS.
- A. Landarani Isfahani, I. Mohammadpoor-Baltork, V. Mirkhani, A. R. Khosropour, M. Moghadam, S. Tangestaninejad and R. Kia, Adv. Synth. Catal., 2013, 355, 957–972 CrossRef.
- R. Luo, R. Tan, Z. Peng, W. Zheng, Y. Kong and D. Yin, J. Catal., 2012, 287, 170–177 CrossRef CAS.
- M. Peng, Y. Chen, R. Tan, W. Zheng and D. Yin, RSC Adv., 2013, 3, 20684–20692 RSC.
- V. Mirkhani, M. Moghadam, S. Tangestaninejad and B. Bahramian, Polyhedron, 2006, 25, 2904–2914 CrossRef CAS.
- M. Zakeri, M. Moghadam, I. Mohammadpoor-Baltork, S. Tangestaninejad, V. Mirkhani and A. R. Khosropour, J. Coord. Chem., 2012, 65, 1144–1157 CrossRef CAS.
- M. Zakeri, M. Moghadam, I. Mohammadpoor-Baltork, S. Tangestaninejad, V. Mirkhani, A. R. Khosropour and M. Alizadeh, Transition Met. Chem., 2012, 37, 45–53 CrossRef CAS.
- A. Sakthivel, J. Zhao, G. Raudaschl-Sieber, M. Hanzlik, A. S. Chiang and F. E. Kühn, Appl. Catal., A, 2005, 281, 267–273 CrossRef CAS.
- P. C. Bakala, E. Briot, L. Salles and J. M. Bregeault, Appl. Catal., A, 2006, 300, 91–99 CrossRef CAS.
- S. M. Bruno, J. A. Fernandes, L. S. Martins, I. S. Gonçalves, M. Pillinger, P. Ribeiro-Claro, J. Rocha and A. A. Valente, Catal. Today, 2006, 114, 263–271 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra18931d |
| This journal is © The Royal Society of Chemistry 2016 |