Development of direct gas injection system for atmospheric-pressure in-solution discharge plasma for plasma degradation and material syntheses†
Motohiro Bannoab,
Kenta Kannoa and
Hiroharu Yui*ab
aDepartment of Chemistry, Faculty of Science, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: yui@rs.kagu.tus.ac.jp
bJST/CREST, 4-1-8 Honcho, Kawaguchi, Saitama 332-0012, Japan
First published on 29th January 2016
Abstract
By applying a high pulsed voltage to a gap between two electrodes placed in a solution, an atmospheric-pressure in-solution glow (ASG) plasma is generated. The ASG plasma is applied in a new material processing method, called solution plasma processing (SPP). In order to accelerate the reaction and to add functions to the synthesized materials, it is important to alter the gas content in the ASG plasma. We developed a direct gas injection system for an ASG discharge cell. When O2, CO2, N2 and Ar gases were injected into the ASG plasma, emission bands due to the derivatives of the injected gases were observed in the emission spectra from the ASG plasma. The electron number density in the ASG plasma was increased by the O2, CO2, and N2 injections, probably due to the enhancements of the α and γ processes by the larger molecular weights than H2O. In addition, the first dielectric breakdown of the solution and the formation of the gas bubble processes gradually disappeared due to the gas injection. When O2 was injected, the amount of ·OH generation was increased. By the enhancement of the ·OH generation, the degradation speed of rhodamine B in the ASG plasma was increased by a factor of two. When Au nanoparticles were synthesized utilizing the ASG plasma, the zeta potential of the Au nanoparticles was increased by about 30% by the O2 injection. The plasma parameters and the reactivity of the ASG plasma can be altered more widely by changing the kind of injected gas and the flow rate.
1. Introduction
When a pulsed high voltage is applied between a gap of two metal electrodes placed in solution, a glow discharge plasma is generated in the solution under atmospheric pressure.1 Atmospheric-pressure in-solution glow (ASG) plasma has attracted much attention as a novel reaction field due to the following properties.1–16 First, a highly inhomogeneous interface is formed between the hot plasma and the cold solution phases. Because of the steep temperature gradient around the interface, it is expected that the interface is applied as a reaction field which is formed with difficulty under atmospheric pressure and room temperature.2 For example, reactions proceeding in the hot plasma are expected to be suddenly quenched by the solution, yielding thermodynamically quasi-stable products. Second, it is known that the ASG plasma contains certain amounts of hydrogen (·H) and hydroxyl (·OH) radicals when the discharge is performed in an aqueous solution.3–6 Since ·H and ·OH exhibit large reduction and oxidation potentials, respectively, the ASG plasma formed in the aqueous solution should be applied as an effective reaction field for reduction and oxidation reactions.In fact, the ASG plasma has been applied to material processing, called solution plasma processing (SPP).1 SPP has been successfully applied to syntheses of metal nanoparticles by the reduction of complexes dissolved into the solution.1,6–10 Recently, carbon nanomaterials have been successfully synthesized using SPP.11–14 SPP also exhibits the ability to sterilize bacteria,3 and decolorize chemical reagents.15,16 These reactions also originate from the large oxidation activity of ·OH.
To apply the ASG plasma more widely in environmental, material, and medical fields, it is essential to control the properties and the functions of the ASG plasma. Since plasma represents ionized gas, the ASG plasma consists of gaseous molecules and ions. Therefore, it should be natural to consider that the properties and functions of the ASG plasma largely depend on the plasma gas composition. It is actually reported that the plasma properties are changed by the gas composition for gliding arc discharge17,18 and for dielectric barrier discharge.19 However, it is difficult to alter the gas composition for the ASG plasma. This is because the ASG plasma is formed in a bubble formed in the solution, and the gas composition in the bubble is dominated by the gaseous solvent species.1,20
Important applications of the ASG plasma are the degradation of chemical compounds and sterilization, owing to the high oxidation activity of water derivatives such as H2O2, O(3P), and ·OH. ·OH has the highest oxidation potential of 2.31 V among the species deriving from water.21 It is expected that the ASG plasma becomes a more oxidative field when the amount of ·OH is increased. The abilities of gliding arc discharge plasma for degradation and sterilization have been investigated when the plasma is formed in an atmosphere containing water vapor.22,23 In these studies, it is reported that the degradation and sterilization activities are enhanced when O2 is used as the carrier gas of the plasma. The enhancements of these activities are due to the increase of the ·OH density in the plasma. Therefore, it is expected that the degradation and sterilization abilities of the ASG plasma due to ·OH should be enhanced if O2 is directly injected into the plasma.
Another important application of the ASG plasma is the synthesis of nanoparticles.1,6–14 Nanoparticles synthesized using ASG plasma exhibit a large dispersion stability. The large dispersion stability may originate from OH groups modified on the particle surfaces by ·OH, which is abundantly generated in the discharge in water. When some of the OH groups are ionized, they exhibit negative electric charges. Because of the repulsion of the negative charges between different particles, the nanoparticles should have minimal aggregation with each other. If the amount of ·OH in the ASG plasma increases with the O2 injection, the dispersion stability of the nanoparticles synthesized should increase.
In the present study, we newly developed an in-solution discharge system with a gas injection system from outside by utilizing a metal pipe as an electrode. Gas was able to be directly injected into the ASG plasma by going through the pipe electrode. We measured the optical emission spectra from the ASG plasma while injecting gas. Emissions from transient species derived from the injected gas in the ASG plasma were successfully observed. We demonstrated SPP with O2-injected ASG plasma for the degradation of rhodamine B and the synthesis of Au nanoparticles and evaluated the degradation efficiency and the dispersion stability of the synthesized Au nanoparticles.
2. Experimental
We introduced a gas injection function to an in-solution discharge system we had constructed.4,20 The images of the apparatus are shown in Fig. 1. Briefly, two electrodes were placed opposite each other in a discharge cell. The gap between the electrodes was set to about 500 μm. The cell was filled with an aqueous solution of NaOH (3 mmol L−1). The electrodes were connected to a power supply (MPS-06K02C-WP1F, Kurita Seisakusho), which supplied bipolar voltage pulses. The amplitude, temporal duration, and repetition rate of the applied pulsed voltage were 1.2 kV, 0.7 μs, and 25 kHz respectively. A tantalum pipe and a tungsten rod were used as the electrodes. The outer and inner diameters of the tantalum pipe were 2 and 1 mm, respectively. The diameter of the tungsten rod was 1 mm. The tantalum pipe electrode was connected to a rubber tube, and the tube was separated into four by a connector. The four branches were respectively connected to mass flowmeters. This system was able to inject four kinds of gases into the ASG plasma while altering the mixing ratio of the gases. In the present study, we injected O2, N2, CO2, and Ar gases into the ASG plasma. We set the flow rate of the gas to 0.1, 0.3, 0.5, 0.7, and 0.9 L min−1 for each gas.The discharge cell was located on the table of an inverted microscope. The emission from the ASG plasma was collected using a reflective objective lens (×15, NA = 0.4) installed in the microscope. Because the bottom of the discharge cell was made from quartz, the emission was collected without being spectrally distorted. The collected emission was introduced to a spectrograph, was dispersed by a grating in the spectrograph and was detected by a streak camera. The obtained emission spectrum was calibrated using the sensitivity of each channel and the wavelength dependence of the sensitivity of the detector. It is also possible that the self-absorption of contents in the plasma contributes to the shapes of the emission lines.24 However, it is concluded from the previous studies on atmospheric-pressure plasmas that the self-absorption does not largely affect the spectral shapes when the diameter of the plasma is 0.1–0.2 μm.25,26 As shown in Fig. 1, the diameter of the ASG plasma is less than 0.3 μm. Therefore, the self-absorption effect should be almost negligible.
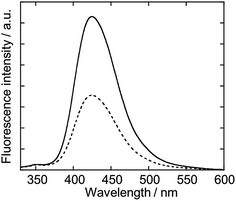
We examined the change in the oxidation reactivity of the ASG plasma by injecting O2. The ASG discharge was performed in an aqueous solution of terephthalic acid, which is a scavenger of ·OH.27 After the discharge, the fluorescence from the hydroxylterephthalic acid, which is a product of the reaction shown in Fig. 2, was measured. The fluorescence spectrum of the solution after the discharge was measured with the excitation wavelength of 310 nm (F-2500, Hitachi High Technologies).
We examined the degradation activity of the ASG plasma with O2 gas injection. The ASG discharge was performed in an aqueous solution of rhodamine B with an initial concentration of 0.4 mmol L−1. The efficiency of the degradation was measured from the temporal change of the absorbance at 550 nm (V-630BIO, JASCO).
We also synthesized Au nanoparticles by utilizing a gold rod electrode instead of the tungsten rod electrode. Au nanoparticles are synthesized by the sputtering of the gold electrode.9 A TEM image of the product solution was obtained and the zeta potential of the synthesized particles was measured (TEM: H-9500, Hitachi High Technologies, zeta potential: Zetasizer ZS (ZEN 3500), Malvern Instruments).
3. Results and discussion
3.1. Optical emission spectrum from the gas-injected ASG plasma
The ASG plasma was successfully generated while directly injecting O2, CO2, N2, and Ar using the developed discharge system. The generated plasma spatially fluctuated because of the gas injection. To identify the contents of the plasma, we measured the optical emission spectrum from the ASG plasma by setting the focus of the objective lens in the microscope to the center of the plasma. The obtained temporally-averaged emission spectra from the plasma are shown in Fig. 3. The flow rate of the gas was set to 0.5 L min−1.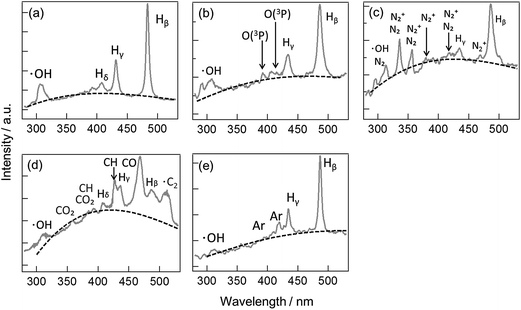 | ||
Fig. 3 Emission spectra from the ASG plasma without the gas injection (a), and with the injections of O2 (b), N2 (c), CO2 (d), and Ar (e). The spectra were obtained with an integration time of 12.8 s, and 320![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pulses were integrated during the time span. The assignments are based on ref. 28–31. The black dotted lines represent the best-fitted curves with the blackbody radiation, eqn (1). 000 pulses were integrated during the time span. The assignments are based on ref. 28–31. The black dotted lines represent the best-fitted curves with the blackbody radiation, eqn (1). | ||
As discussed later, the electron number density in the plasma increased as the gas flow rate became high, and the increase of the density was almost saturated when the flow rate was higher than 0.5 L min−1. We assumed that the effect of the gas injection was maximized and almost saturated when the gas flow rate was higher than this value.
As shown in Fig. 3, several emission bands are observed in the emission spectrum in the wavelength range between 280 and 540 nm. An emission band due to ·OH is observed at around 310 nm, and emission bands due to ·H at 434 and 486 nm are commonly observed for the ASG plasma with the four different gas injections. These emission bands are also observed for the ASG plasma without gas injection.4 In addition, as shown in Fig. 3, several additional bands are observed when the gas is injected: bands at 390 and 420 nm for O2, at 360, 390, 430, 460, and 510 nm for CO2, at 340, 350, 380, 410, and 460 nm for N2, and at 360 and 420 nm for Ar. These bands are assigned to the emissions from transient species derived from the injected gas species.28–31 For example, the emission bands at 390 and 420 nm with the O2 injection are assigned to the triplet oxygen atom (O(3P)).28
From these results, it is concluded that the injected gas is taken into the ASG plasma reaction field, and the transient species derived from the injected gas are expected to work as the reactive species in the reaction field.
3.2. Change of plasma parameters caused by gas injection
To discuss the changes of the properties in the ASG plasma reaction field caused by the gas injections, we estimated two parameters of the plasma by analyzing the optical emission spectrum. One was blackbody temperature, Tb, determined by the curve fitting of the continuous emission observed in the wavelength range between 270 and 530 nm. The blackbody emission is also observed for the ASG plasma without the gas injection.4 Because the Tb value in the original ASG plasma increased in the early time domain before the delay time of 0.2 μs, we have suggested that the electronic temperature contributes to the Tb value to a certain extent.4 For the present gas-injected ASG plasma, the average Tb value was determined with the fitting of the temporally averaged emission spectrum using the following equation
 | (1) |
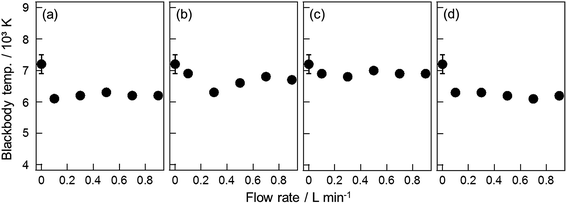 | ||
| Fig. 4 Effect of the flow rate of the gas injection on the average blackbody temperature. The injected gases are O2 (a), N2 (b), CO2 (c), and Ar (d). | ||
As shown in Fig. 4, Tb decreases by about 1000 to 2000 K, depending on the gas flow rate. This decrease of the Tb value should indicate that the average temperature of the components contained in the ASG plasma decreases because of the injection of cold gas at room temperature.
We also examined the electron number density, ne, from the emission spectra. It is reported that the bandwidth of the Hβ emission at the wavelength of 486 nm is broadened by several factors: Stark broadening, Doppler broadening, and pressure broadening effects.30 Under the present experimental conditions, the contributions from the Doppler and pressure broadening effects to the width of the Hβ emission linewidth are estimated to be less than 0.1 nm, while the width of the obtained Hβ emission line is more than several nanometers.30 Therefore, the width of the Hβ emission line predominantly reflects the contribution from the Stark broadening. The Stark broadening results from the electric field around ·H caused by the moving free electrons in the plasma. The linewidth of the Hβ emission, Δλ, is related to ne by the following equation
| ne = 1.04 × 1016 × (Δλ)1.497 | (2) |
We applied eqn (2) to the obtained spectrum of the Hβ emission line shown in Fig. 3, and estimated the temporal average of ne for the ASG plasma with the gas injection. The estimated ne is plotted against the gas flow rate in Fig. 5. As shown in Fig. 5, ne increases as the flow rate of O2, CO2, and N2 increases, while it decreases as the flow rate of Ar increases.
 | ||
| Fig. 5 Effect of the flow rate of the gas injection on the electron number density. The injected gases are O2 (cross), N2 (diamond), CO2 (square), and Ar (circle). | ||
The change in the electron number density caused by gas injection should result from electron generation processes by the injected gas molecules. The electrons in the ASG plasma are generated by the collisions between the positively charged ions accelerated by the applied voltage and other particles (α process) and between the ions and the cathodic electrode (γ process).32 When the gas is not injected, the dominant components in the ASG plasma are H2O and its derivatives. On the other hand, when the gas is injected, the ASG plasma contains the injected gas molecules and their derivatives. The molecular weights of the injected gases are larger than that of H2O. Therefore, the kinetic impulses of the collisions become large due to the injections of the gases. The electrons should be effectively supplied in the plasma due to the large kinetic impulses.
When Ar gas is injected, however, the electron number density decreases. Ar is an inert gas, having a large ionization energy of about 1500 kJ mol−1.33 It is considered that Ar is rarely ionized in the ASG plasma, and thus Ar contributes little to the electron generation processes. In Fig. 3(e), emission bands due to ionized Ar, or Ar+, are not observed. This result also indicates that Ar is rarely ionized in the ASG plasma. When Ar is injected, the density of H2O in the ASG plasma is reduced, and the number of electrons generated by the α and γ processes related to the H2O molecules and their derivatives becomes consequently small.
It is concluded that the electron number density is continuously controllable in the range between 1016 and 1017 cm−3 by altering the injected gas species and the flow rate. This feature of the gas-injected ASG plasma may be applicable for altering the parameters of the plasma reaction field, such as the collision frequencies between the electrons and the transient reactive particles of the reaction.
3.3. Temporal evolution of the emissions from radicals
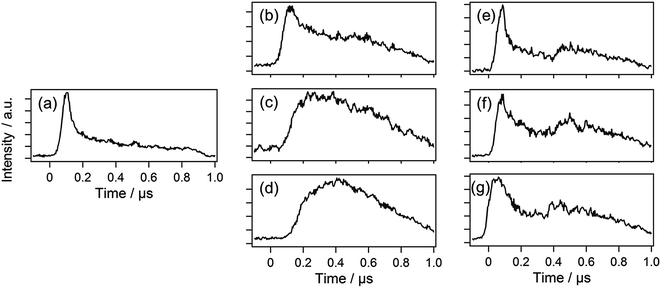
The temporal evolution of the emission from ·OH is shown in Fig. 6. When the gas is not injected, the temporal evolution can be separated into two parts (Fig. 6(a)). The first part is the delay time region (0–0.2 μs). In this part, the emission intensity rises, and it immediately decays. The second part is the time region after the delay time of 0.2 μs. In this part, the emission intensity again rises.We have reported that the ASG plasma is generated in a bubble formed in the solution.4,20 When the generation and sustaining mechanisms of the ASG plasma are considered, the first part should represent the processes for the dielectric breakdown and formation of the ASG plasma. In this part, the water vapor bubble is also formed at the gap between the electrodes in the solution. In the second part, the plasma should be formed again by the applied voltage. In this part, charged particles, or electrons and ions, in the ASG plasma are accelerated by the applied voltage, and they collide with other particles. The particles in the ASG plasma are excited by the collisions. The intensity rises of the emissions from the radicals should represent this excitation process.
As shown in Fig. 6, the two parts becomes gradually inseparable as the O2 flow rate increases. Similar changes were also observed when N2 or CO2 was injected. In the discussion above, we have assigned the first part to the dielectric breakdown and the formation of the water vapor bubble. However, when the gas is injected from outside, the ASG plasma can be generated in the bubbles of the injected gas formed in the solution. Therefore, the first part, which represents the dielectric breakdown and the bubble formation, is unnecessary for the growing of the ASG plasma by gas injection. Therefore, probably only the second part appears in the temporal evolution.
Note that the first and second parts are clearly separable when Ar is injected as shown in Fig. 6. Ar is a highly inert gas, and it is hardly ionized. When the voltage is applied to an Ar bubble, the ASG plasma should not be formed in the Ar gas. Instead, the ASG plasma is formed in a similar way to that formed without the gas injection. When the voltage is applied to the gap between the electrodes, the water in the gap is vaporized due to the dielectric breakdown, and the water vapor is taken into the Ar bubble. The first peak is attributed to this breakdown process. After the breakdown process, the ASG plasma is formed in the Ar/water bubble, resulting in the second emission peak.
We have reported that the electron number density of the ASG plasma is higher by a factor of about ten for the time region of 0–0.2 μs after the beginning of the emission than the value averaged for 1 μs.4 The high electron number density should result from the dielectric breakdown of the solution between the electrodes. The reactant, intermediate, and product molecules may be dissociated by the collisions with electrons in the plasma reaction field. It is expected that the contributions from the frequent collisions with the high-energy electrons in the first dielectric breakdown process may be partly inhibited by the gas injection.
3.4. Enhancement of ·OH generation by O2 injection and its application to material syntheses
Several schemes are suggested for the generation of ·OH when O2 gas exists in a plasma reaction field.23 Note that O2 is dissociated into two O(3P) by collisions with free electrons, and the resultant O(3P) is a source of ·OH generation pathways.23 As shown in Fig. 3(b), the emission from O(3P) is observed at the wavelengths of 390 and 420 nm. When O2 is injected into the ASG plasma, the relative intensity of the O(3P) emission is increased. Therefore, it is suggested that the generation of O(3P) is enhanced by the O2 gas injection, and the generated O(3P) is used as the source of ·OH generation. From these results, possible pathways of ·OH generation in the ASG plasma are suggested.
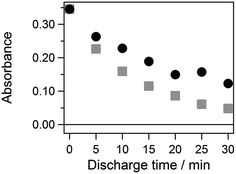 | ||
| Fig. 8 Effect of the ASG discharge time on the absorbance of rhodamine B. O2 gas was not injected (circle) and was injected (square). | ||
To discuss the degradation efficiency more quantitatively, the effect of time was fitted with an exponential function, and the decay rates were estimated. The decay rates were estimated as 0.032 min−1 and 0.065 min−1 for the discharge without and with the oxygen injection, respectively, by fitting with an exponential function. The degradation speed of rhodamine B was increased by a factor of two by the oxygen injection. It is concluded that the direct oxygen injection certainly enhances the oxidation reactivity for the ASG plasma, probably owing to the increase of the ·OH generation.
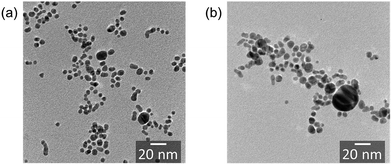 | ||
| Fig. 9 TEM images of Au nanoparticles synthesized using the ASG plasma. O2 gas was not injected (a) and was injected (b). The average diameter is 5.47 ± 1.70 nm for (a) and 7.70 ± 3.64 nm for (b). | ||
It is reported that when an Au rod is utilized as the electrode for the ASG discharge, Au fragments are released from the surface of the tip of electrodes to the ASG plasma by the collisions between the electrode surface and cationic species accelerated by the applied voltage.9 When O2 was injected into the ASG plasma, the electron number density increased as shown in Fig. 5. Since the total electric charge in plasma is kept almost neutral, the number of cations in the ASG plasma should also increase accordingly with the increase in electrons. Therefore, the amounts of Au fragments released to the ASG plasma should be increased by the O2 injection. Because of the increase in the number of the Au fragments, the fragments should collide with each other more frequently, yielding nanoparticles with larger diameters.
We also examined the zeta potential of the Au nanoparticles. The zeta potential was changed from −58 mV to −73 mV by the O2 injection. As mentioned in Henry’s law, the zeta potential measured by electrophoresis depends on the particle size.34 Thus, as an origin of the increase in zeta potential, the size effect of the synthesized particle is considerable at first. As shown in Fig. 9, the particle diameter was changed from 5.5 nm to 7.7 nm (ca. 40% increase) by the O2 injection. The dependence of the zeta potential on the particle diameter can be numerically calculated based on Henry’s law.34 The solution used in the present study was an aqueous solution of NaOH with the concentration of 3 mmol L−1. Under this concentration, the Debye–Hückel parameter κ, that is, the thickness of the electric double layer, is estimated as about 8 nm. Therefore, the diameter of the particle obtained in the present experiment is the same order as κ. Under these conditions, even when the increase in particle diameter reaches up to about 40%, it is estimated that the zeta potential increases only by less than 5%.34 Therefore, the remarkable increase of the zeta potential by 30% caused by the O2 injection is not fully explained only by the increase of the particle diameter.
Another probable, but main origin of the increase in zeta potential is the modification of the particle surfaces by OH groups. As mentioned above, the ·OH generation in the ASG plasma was enhanced by the O2 injection. The enhanced surface modification by ·OH should increase the zeta potential by a large extent.
From these results, it is concluded that the ASG plasma can be applied more effectively to both environmental purification and material syntheses by altering the chemical compositions of the reaction field using the direct gas injection system developed here. Further applications are also expected by varying the combination of the kind and amount of the gases introduced through the injection system.
4. Conclusion
We developed a direct gas injection system for an ASG discharge cell. When O2, CO2, N2 and Ar gases were injected into the ASG plasma, the emission bands due to the derivatives of the injected gases were observed in the emission spectra from the ASG plasma. The electron number density in the ASG plasma was increased by the O2, CO2, and N2 injections, probably due to the enhancements of the α and γ processes by the larger molecular weights than H2O. In addition, the first dielectric breakdown of the solution and the formation of the gas bubble process became no longer evident due to the gas injection. When O2 was injected, the amount of ·OH generation was increased. By the enhancement of the ·OH generation, the ASG plasma reaction field exhibited a higher ability for the degradation of rhodamine B. In addition, the dispersion stability of Au nanoparticles synthesized in the ASG plasma was increased by the O2 injection. It is revealed that the plasma parameters and the reactivity of the ASG plasma can be changed more widely by changing the kind of injected gas and the flow rate.References
- O. Takai, Pure Appl. Chem., 2008, 80, 2003 CrossRef CAS.
- N. Saito, J. Hieda and O. Takai, Thin Solid Films, 2009, 518, 912 CrossRef CAS.
- N. Andreeva, T. Ishizaki, P. Baroch and N. Saito, Jpn. J. Appl. Phys., 2012, 51, 126201 CrossRef.
- M. Banno, K. Kanno, Y. Someya and H. Yui, Jpn. J. Appl. Phys., 2015, 54, 066101 CrossRef.
- C. Miron, M. A. Bratescu, N. Saito and O. Takai, J. Appl. Phys., 2011, 109, 123301 CrossRef.
- M. A. Bratescu, S.-P. Cho, O. Takai and N. Saito, J. Phys. Chem. C, 2011, 115, 24569 CAS.
- S.-P. Cho, M. A. Bratescu, N. Saito and O. Takai, Nanotechnology, 2011, 22, 455701 CrossRef PubMed.
- M. A. Bratescu and N. Saito, J. Phys. Chem. C, 2013, 117, 26804 CAS.
- M. A. Bratescu, O. Takai and N. Saito, J. Alloys Compd., 2013, 562, 74 CrossRef CAS.
- X. Hu, X. Zhang, X. Shen, H. Li, O. Takai and N. Saito, Plasma Chem. Plasma Process., 2014, 34, 1129 CrossRef CAS.
- H. Lee, M. A. Bratescu, T. Ueno and N. Saito, RSC Adv., 2014, 4, 51758 RSC.
- D. Kim, O. L. Li, P. Pootawang and N. Saito, RSC Adv., 2014, 4, 16813 RSC.
- G. Panomsuwan, S. Chiba, Y. Kaneko, N. Saito and T. Ishizaki, J. Mater. Chem. A, 2014, 2, 18677 CAS.
- G. Panomsuwan, N. Saito and T. Ishizaki, J. Mater. Chem. A, 2015, 3, 9972 CAS.
- P. Baroch, N. Saito and O. Takai, J. Phys. D: Appl. Phys., 2008, 41, 085207 CrossRef.
- M. A. Bratescu, J. Hieda, T. Umemura, N. Saito and O. Takai, J. Vac. Sci. Technol., A, 2011, 29, 031302 Search PubMed.
- M. Moravej, X. Yang, M. Barankin, J. Penelon, S. E. Babayan and R. F. Hicks, Plasma Sources Sci. Technol., 2006, 15, 204 CrossRef CAS.
- L. Yu, X. Li, X. Tu, Y. Wang, S. Lu and J. Yan, J. Phys. Chem. A, 2010, 114, 360 CrossRef CAS PubMed.
- A. A. Abdelaziz, T. Seto, M. Abdel-Salam and Y. Otani, Plasma Chem. Plasma Process., 2014, 34, 1371 CrossRef CAS.
- H. Yui, Y. Someya, Y. Kusama, K. Kanno and H. Takakuwa, Bunseki Kagaku, 2013, 62, 19 CrossRef CAS.
- W. H. Koppenol, D. M. Stanbury and P. L. Bounds, Free Radicals Biol. Med., 2010, 49, 317 CrossRef CAS PubMed.
- J. Yan, C. Du, X. Li, X. Sun, M. Ni, K. Cen and B. Cheron, Plasma Sources Sci. Technol., 2005, 14, 637 CrossRef CAS.
- R. Peyrous, P. Pignolet and B. Held, J. Phys. D: Appl. Phys., 1989, 22, 1658 CrossRef CAS.
- I. Hutchinson, Principles of Plasma Diagnostics, Cambridge University Press, Cambridge, 2nd edn, 2002 Search PubMed.
- P. Bruggeman, D. Schram, M. Á. González, R. Rego, M. G. Kong and C. Leys, Plasma Sources Sci. Technol., 2009, 18, 025017 CrossRef.
- R. M. van der Horst, T. Verreycken, E. M. van Veldhuizen and P. J. Bruggeman, J. Phys. D: Appl. Phys., 2012, 45, 345201 CrossRef.
- R. W. Matthews, Radiat. Res., 1980, 83, 27 CrossRef CAS PubMed.
- J. E. Sansonetti, W. C. Martin and S. L. Young, Handbook of Basic Atomic Spectroscopic Data, Atomic Spectra Database, NIST Standard Reference Database Number 108, ed. P. J. Linstrom and W. G. Mallard, National Institute of Standards and Technology, Gaithersburg, MD, August 15, 2015, p. 20899, http://www.nist.gov/pml/data/handbook Search PubMed.
- M. Kraus, W. Egli, K. Haffner, B. Eliasson, U. Kogelschatz and A. Wokaun, Phys. Chem. Chem. Phys., 2002, 4, 668 RSC.
- C. O. Laux, T. G. Spence, C. H. Kruger and R. N. Zare, Plasma Sources Sci. Technol., 2003, 12, 125 CrossRef CAS.
- A. Bogaerts, R. Gijbels and J. Vlcek, Spectrochim. Acta, Part B, 1998, 53, 1517 CrossRef.
- Y. P. Raizer, Gas Discharge Physics, Springer, Berlin, 1991 Search PubMed.
- I. Velchev, W. Hogervorst and W. Ubachs, J. Phys. B: At., Mol. Opt. Phys., 1999, 32, L511 CrossRef CAS.
- H. Ohshima, J. Colloid Interface Sci., 1994, 168, 269 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra18836a |
| This journal is © The Royal Society of Chemistry 2016 |