Radioprotective effects of active compounds of Acanthopanax senticosus from the Lesser Khingan Mountain range in China
Wei Songab,
Jinming Shic,
Denis Baranenkod,
Jing Jingab and
Weihong Lu*ab
aInstitute of Extreme Environment Nutrition and Protection, Harbin Institute of Technology, Harbin 150090, China. E-mail: weisong@hit.edu.cn; lwh@hit.edu.cn; jingjing@hit.edu.cn; Fax: +86-451-86289633; Tel: +86-451-86289633
bDepartment of Food Science and Engineering, Harbin Institute of Technology, Harbin 150090, China
cCollege of Life Science, Northeast Forestryl University, Harbin 150090, China. E-mail: shijinming8888@163.com
dInstitute of Refrigeration and Biotechnologies, ITMO University, Saint-Petersburg, Russia. E-mail: denis.baranenko@niuitmo.ru
First published on 9th December 2015
Abstract
Bioactive compounds including polysaccharides, flavones, syringin and eleutheroside E were extracted from wild Acanthopanax senticosus to obtain purities of 88.4% ± 3.2%, 90.8% ± 2.0%, 92.5% ± 1.5% and 82.7% ± 4.7% respectively. In vitro antioxidant activities and in vivo anti-radiation activities of the compounds were investigated and compared. The results demonstrated that polysaccharides and flavones extracted from A. Senticosus were more effective than syringin and eleutheroside E in their radical scavenging activity in vitro. In vivo studies showed that polysaccharides and flavones were also effective in protecting mice from heavy ion radiation induced tissue oxidative damage. Furthermore, the activities of polysaccharides and flavones in repressing expression changes of radiation response proteins including heat shock protein, disulfide-isomerase and glutathione S-transferase, were also identified by our results. These radioprotective effects were more significant when polysaccharides and flavones were administered together.
1. Introduction
Radiation exposure can cause many kinds of disease by increasing oxidative pressure in living organisms. Among all the possible sources of radiation, heavy ion radiation is the most harmful in terms of risks to humans, as it is usually more than 10-fold more effective in causing cell damage than other sources of radiation such as X-rays, γ-rays, or electrons.1,2 In recent decades, chemicals and functional compounds derived from natural products have been the focus of investigation with the aim of identifying new radioprotective medicines and foods that may improve human health.3,4 Amongst these natural products, some traditional Chinese medicines such as herbal drugs have been used for millennia. Nowadays, more and more herbal drugs are being investigated to identify the functional compounds within them and to determine their biological activities.5 The potential of these herbal drugs to be exploited as new functional foods, such as anti-cancer and anti-radiation foods, is becoming the subject of increased attention.Acanthopanax senticosus (AS) is one of the most well known traditional Chinese herbal drugs which is constantly spread and widely cultivated in North Asia. AS has been used for the treatment of many different kinds of disease and disease symptoms, such as hypertension,6 hepatitis,7 inflammation8 and ischemic disease.9 In recent years it has been revealed that the main active compounds in AS are eleutheroside, flavones and polysaccharides. The biological activity of these compounds has been widely investigated in mammals, plants and insects.10 AS extract has been shown to enhance gut health in weaning piglets,11 exert protective effects on gut immunity of Drosophila,12 and to reduce oxidative stress.13 These findings suggest that there is potential for AS and its active compounds to be explored as health care products with anti-radiation activities. However, to date, little research attention has been given to this subject.
The Lesser Khingan Mountain range is one of the three largest forested areas in China and is located in the transitional zone between the boreal and temperate forests. More than half of the wild AS used by the Chinese comes from the Lesser Khingan range. Acanthopanax senticosus originating from the Lesser Khingan range (LAS) has been most widely used because it grows in the natural environment. In the present study, we separated the active compounds of polysaccharides, flavones, syringin and eleutheroside E from LAS. The activities of in vitro anti-oxidative and in vivo anti-radiation properties of these compounds were investigated and compared.
2. Experimental section
2.1. Chemicals
Syringin, eleutheroside E, D-glucose and rutin were purchased from National Institutes for Food and Drug Control (Beijing, China). α-Cyano-4-hydroxycinnamic acid (CHCA) was purchased from SigmaeAldrich (Buchs, Switzerland). Ferrous chloride, polyoxyethylene sorbitan monolaurate (Tween 20), a-tocopherol, 1,1-dipheny-l-2-picrylhydrazyl (DPPH), 3-(2-pyridyl)-5,6-bis-(4-phenyl-sulfonic acid)-1,2,4-triazine (ferrozine), nicotinamide adenine dinucleotide (NADH), and trichloracetic acid (TCA) were purchased from Sigma. The reagents and kits for detecting enzyme activation were purchased from Nanjing Jiancheng Bioengineering Institute of China. All others unlabelled chemicals and reagents were analytical grade.2.2. Plant material
Acanthopanax senticosus (LAS) was collected in Lesser Khingan Mountain, Heilongjiang, China, in September 2010. The AS was identified by Prof. Yu Jiabin (Herb Identification and Medicinal Plant Teaching and Research Section, Heilongjiang University of Chinese Medicine). The 4 year old LAS were chosen randomly. The root, stem, leave and fruit of LAS was separated and stored at −80 °C after freeze-dried.2.3. Preparation of active compounds from Lesser Khingan Mountain range derived Acanthopanax senticosus (LAS)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 40
1, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20
1, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10
1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5
1, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Component 1 (from 5
2). Component 1 (from 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 elution) and component 2 (from 1
1 elution) and component 2 (from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 elution) were collected to obtain syringin and eleutheroside E respectively after reducing pressure to recover the solvent. The compounds were then dried in vaccum at 40 °C.
2 elution) were collected to obtain syringin and eleutheroside E respectively after reducing pressure to recover the solvent. The compounds were then dried in vaccum at 40 °C.Dried flavonoid extracts were smashed and sieved using a 20 mesh sieve. A total of 0.5 g of the resulting fine powder was then weighed and dissolved in ethanol. After circulation reflux, filtration and vacuum concentration, the residue was dissolved in methanol and diluted to 100 ml. 0.1 ml of the residue solution was measured accurately and dissolved in methanol to 20 ml. The concentration of total flavones was then detected at 257 nm by UV spectrophotometer (Shimadzu-UV-2450, Shanghai, China).19 Rutin solutions were used for constructing the calibration curve (y = 30.69x + 0.0274; R2 = 0.9992). Total flavonoid content of the extract was expressed as grams of rutin equivalents per 100 g of dried LAS (%).
2.4. Measurement of in vitro antioxidant capacity
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, and the resulting mixture was incubated for 6 min at room temperature. The absorbance was measured at 730 nm. The scavenging activity on ABTS radical was calculated against a control sample.
20, and the resulting mixture was incubated for 6 min at room temperature. The absorbance was measured at 730 nm. The scavenging activity on ABTS radical was calculated against a control sample.2.5. Animal experiments
A total of 72 male mice of 6–8 weeks old, weighing 20.0 ± 5 g, were purchased from the Health and Anti-epidemic Service, Lanzhou, China, and maintained under conditions of controlled temperature (23 ± 2 °C) and humidity (50 ± 5%), and a 12 h light/dark cycle. The mice were allowed at least 1 week to adapt to the environment before being used for experiments. Mice were randomly divided into 12 groups (n = 6 per group). These groups comprised of: normal control group, irradiated control group, polysaccharides (5 mg per day) treatment group, polysaccharides (10 mg per day) treatment group, flavones (5 mg per day) treatment group, flavones (10 mg per day) treatment group, eleutheroside E (5 mg per day) treatment group, eleutheroside E (10 mg per day) treatment group, syringin (5 mg per day) treatment group, syringin (10 mg per day) treatment group, polysaccharides (1.5 mg per day) plus flavones (3.5 mg per day) treatment group, and polysaccharides (3 mg) plus flavones (7 mg) treatment group.The active compound samples to be tested were dissolved in normal saline (NS). Mice were then exposed daily to aqueous solutions of the active compounds at the above described dosing levels over a two week period. The mice in the control groups were given NS daily on the basis of equal volume. All mice, except those from the normal control group, were irradiated by 12C6+ with the mean linear energy transfer (LET) value of 62.2 keV μm−1 at a dose of 2 Gy and a dose rate of 0.1 Gy min−1. Each mouse was placed in a separate plastic container (20 cm × 20 cm × 100 cm), and exposed to head irradiation. Mice of each group were euthanized by cervical dislocation at three days after irradiation. The procedures of the experiment were strictly performed according to the generally accepted international rules and regulations.
2.6. MDA content and SOD activity
Malondialdehyde (MDA) content of mice tissue homogenates was assessed spectrophotometrically using the thiobarbituric acid test.24 Superoxide dismutase (SOD) (EC 1.15.1.1) activity was measured spectrophotometrically based on inhibition of the photochemical reduction of nitroblue tetrazolium.25,262.7. Two-dimensional gel electrophoresis (2-DE) analysis
3. Results and discussion
3.1. Content detection of active compounds from LAS
Water soluble polysaccharides were extracted from the root, stem, leaves and fruits of LAS. The extraction yield of the polysaccharides across the different LAS plant tissues was 7.5%–17.1%. The total carbohydrate content in the extracted polysaccharides was determined by the phenol–sulfuric acid method. The yield of ethanol extract across the different LAS plant tissues was 33.5%–45.3%. The yields of flavones, syringin and eleutheroside E from the ethanol extracts of the different LAS plant tissues were determined by spectrophotometric method or HPLC. The content of each active compound in each of the different LAS tissue types is listed in Table 1. In each case, the yield was calculated according to the dry biomass of root, stem, leaves and fruits of LAS respectively. As shown in Table 1, the contents of the active compounds in the root and stem are generally higher than those in the leaves and fruit.| Compounds | Content of the active compounds (%) | |||
|---|---|---|---|---|
| Tissue of Lesser Khingan Mountain Acanthopanax senticosus | ||||
| Root | Stem | Leaf | Fruit | |
| Polysaccharides | 6.05 ± 0.25 | 8.09 ± 0.37 | 4.12 ± 0.19 | 3.08 ± 0.13 |
| Flavones | 14.25 ± 0.66 | 9.26 ± 0.45 | 4.02 ± 0.17 | 9.58 ± 0.50 |
| Syringin | 1.44 ± 0.06 | 1.86 ± 0.08 | 0.09 ± 0.01 | 0.02 ± 0.001 |
| Eleutheroside E | 3.17 ± 0.12 | 4.14 ± 0.25 | 0.18 ± 0.01 | 0.04 ± 0.002 |
3.2. Isolation and purification of active compounds from LAS
The purity of the polysaccharides extracted from the different tissues of LAS was calculated to be 88.4% ± 3.2%. Flavones, syringin and eleutheroside E were isolated from EELAS by the method of column chromatography. Flavones were separated by macroporous adsorption resin. The purity was calculated to be 90.8% ± 2.0%, detected by the spectrophotometric method. Syringin and eleutheroside E were separated and purified by silica gel column chromatography. The content of each was detected by HPLC using syringin and eleutheroside E as the respective standards. The purity of the extracted syringin and eleutheroside E was 92.5% ± 1.5% and 82.7% ± 4.7% respectively.3.3. In vitro antioxidant activities of active compounds from LAS
Recently, a wide range of spectrophotometric assays have been adopted to measure the antioxidant capacity of active compounds from drugs and foods. The most popular methods used have been the ABTS assay and DPPH assay.31–33 In the present study, extracted active compounds including syringin, eleutheroside E, flavones and polysaccharides were diluted to appropriate concentrations to evaluate their anti-oxidative activity in terms of their DPPH and ABTS radical scavenging activity.In the case of the DPPH assay, as expected, the active compounds exhibited radical scavenging activities in a concentration dependent manner (Fig. 1A). When the concentration was below 5 mg ml−1, the radical scavenging activities of syringin, polysaccharides and flavones all increased significantly with the increasing concentration. At 5 mg ml−1, the radical scavenging activities of polysaccharides, flavones, syringin and eleutheroside E were 72.3, 83.0, 58.3 and 13.2% respectively. The IC50 values of the extracted polysaccharides, flavones and syringin were 1.4 mg ml−1, 2.2 mg ml−1 and 4.2 mg ml−1, respectively. These results demonstrate that, compared with syringin and eleutheroside E, polysaccharides and flavones were more effective in DPPH radical scavenging (Fig. 1A).
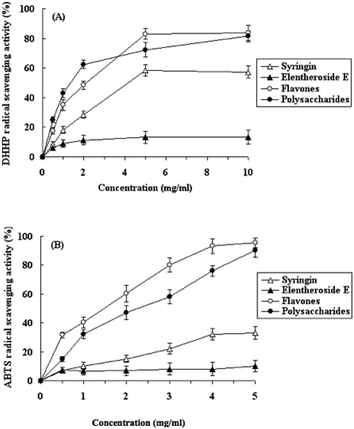 | ||
| Fig. 1 DPPH (A) and ABTS (B) radical scavenging activities of polysaccharides, flavones, syringin and eleutheroside E extracted from LAS. Each value is the mean ± SD of triplicate measurements. | ||
In the case of the ABTS assay, the results were similar to those of the DPPH assay. As shown in Fig. 1B, the radical scavenging activities of the active compound extracts increased in a concentration dependent manner. The IC50 values of the extracted polysaccharides and flavones were 2.3 mg ml−1 and 1.5 mg ml−1, respectively. The results show that the polysaccharides and flavone extracts exhibited higher radical scavenging activities than those of syringin and eleutheroside E. However, these results must be interpreted with some caution as it is possible that a certain amount of impurities may have contributed to the observed antioxidant activities.
3.4. In vivo antioxidant activities of active compounds from LAS
| Group | Dose (mg d−1) | Content of MDA (nmol mgprot−1) | ||||
|---|---|---|---|---|---|---|
| Liver | Spleen | Kidney | Brainstem | Testicle | ||
| a P < 0.001 (compared with normal control).b P < 0.05 (compared with radiation control).c P < 0.01 (compared with radiation control).d Polysaccharides plus flavones treatment group. | ||||||
| Normal control | 6.83 ± 0.72 | 4.85 ± 0.54 | 3.75 ± 0.85 | 3.23 ± 0.41 | 2.58 ± 0.66 | |
| Radiation control | 15.98 ± 2.21a | 10.27 ± 1.96a | 9.24 ± 2.18a | 8.33 ± 3.56a | 6.72 ± 1.13a | |
| Polysaccharides | 10 | 7.12 ± 0.85b | 5.69 ± 1.57b | 4.10 ± 1.33b | 4.33 ± 1.44b | 3.85 ± 1.33b |
| 5 | 9.31 ± 0.98b | 7.54 ± 2.13b | 6.56 ± 2.10b | 5.45 ± 1.69b | 4.13 ± 2.13b | |
| Flavones | 10 | 7.94 ± 1.17b | 5.14 ± 1.67b | 4.66 ± 1.46b | 4.24 ± 1.30 | 3.56 ± 1.50b |
| 5 | 10.19 ± 1.58c | 8.88 ± 1.20b | 7.34 ± 2.82b | 6.56 ± 3.10b | 4.55 ± 1.66b | |
| Eleutheroside E | 10 | 12.98 ± 1.73 c | 9.33 ± 2.22c | 8.08 ± 2.22 | 6.46 ± 5.30c | 5.11 ± 1.33b |
| 5 | 14.22 ± 2.17 | 9.91 ± 1.90 | 8.56 ± 2.14 | 7.64 ± 4.98 | 5.89 ± 2.14 | |
| Syringin | 10 | 13.43 ± 2.49 | 9.34 ± 3.60c | 8.39 ± 2.45 | 6.58 ± 3.20c | 5.98 ± 3.23 |
| 5 | 15.44 ± 3.18 | 10.90 ± 2.90 | 9.13 ± 3.68 | 7.88 ± 4.29 | 6.65 ± 2.10 | |
| P + Fd | (3 + 7) | 7.15 ± 0.64b | 5.09 ± 1.18b | 4.18 ± 1.37b | 3.98 ± 1.37b | 3.44 ± 1.23b |
| (1.5 + 3.5) | 8.55 ± 0.89b | 7.14 ± 1.88b | 5.99 ± 1.83b | 5.17 ± 1.58b | 4.24 ± 1.85b | |
| Group | Dose | SOD activity (U mgprot−1) | ||||
|---|---|---|---|---|---|---|
| Liver | Spleen | Kidney | Brainstem | Testicle | ||
| a P<0.001 (compared with normal control).b P<0.05 (compared with radiation control).c P<0.01 (compared with radiation control).d Polysaccharides plus flavones treatment group. | ||||||
| Normal control | 433.30 ± 33.20 | 262.50 ± 16.25 | 153.25 ± 7.58 | 96.45 ± 8.68 | 100.88 ± 8.30 | |
| Radiation control | 253.55 ± 19.23a | 180.60 ± 18.30a | 88.56 ± 6.53 a | 43.45 ± 6.63a | 68.54 ± 9.12a | |
| Polysaccharides | 10 mg d−1 | 390.35 ± 18.50b | 238.20 ± 23.85b | 123.33 ± 14.41b | 87.20 ± 7.25b | 91.78 ± 8.50b |
| 5 mg d−1 | 350.45 ± 22.31b | 205.22 ± 18.33b | 100.24 ± 9.30b | 71.33 ± 8.90b | 86.45 ± 7.12b | |
| Flavones | 10 mg d−1 | 361.45 ± 26.76b | 255.80 ± 25.30b | 115.22 ± 13.00b | 88.79 ± 5.36b | 85.34 ± 10.10b |
| 5 mg d−1 | 328.80 ± 17.20b | 224.50 ± 20.80b | 95.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.45c 8.45c |
72.57 ± 7.75b | 78.36 ± 6.47c | |
| Eleutheroside E | 10 mg d−1 | 256.19 ± 31.66 | 218.44 ± 19.33b | 98.69 ± 13.25 | 51.47 ± 9.13c | 74.85 ± 9.70 |
| 5 mg d−1 | 225.33 ± 32.12 | 195.90 ± 14.88 | 87.44 ± 12.30 | 46.35 ± 8.53 | 70.15 ± 9.41 | |
| Syringin | 10 mg d−1 | 280.24 ± 42.20 | 208.44 ± 20.22c | 90.77 ± 15.41 | 47.10 ± 5.39 | 79.63 ± 7.50 |
| 5 mg d−1 | 268.45 ± 48.33 | 195.52 ± 18.65 | 92.38 ± 9.78 | 49.65 ± 9.38 | 71.44 ± 10.22 | |
| P + Fd | (3 + 7) mg d−1 | 411.53 ± 15.80b | 258.25 ± 22.44b | 141.05 ± 16.13b | 89.05 ± 8.20b | 90.21 ± 8.45b |
| (1.5 + 3.5) mg d−1 | 385.45 ± 21.16b | 235.22 ± 19.64b | 118.90 ± 12.55b | 77.59 ± 9.90b | 85.60 ± 9.34b | |
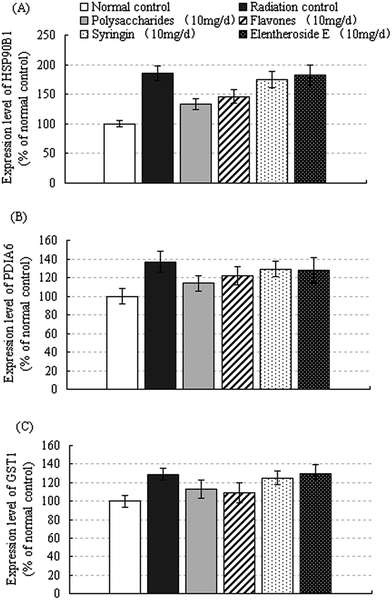
3.5. Effects of active compounds from LAS on proteins in mice liver after radiation
Mouse liver proteins were separated by 2-DE. A total of 18 gels (3 individuals × 6 treatment groups) were produced. The 6 treatment groups included: normal control group; irradiated control group; polysaccharide (10 mg) treatment group; flavone (10 mg) treatment group; eleutheroside E (10 mg) treatment group; syringin polysaccharides and flavones (10 mg, 3 mg and 7 mg respectively) treatment group. Approximately 300 spots in each gel were detected by the automated spot detection algorithm across the 18 gels. Proteins associated with each gel spot were identified by MALDI mass spectrometry analysis. Three proteins including heat shock protein 90B1 (HSP90B1), disulfide-isomerase A6 (PDIA6) and glutathione S-transferase pi (GSTP1) were identified as the significantly changed spots. The expression levels of the above 3 proteins in each treatment group were compared in Fig. 2.As shown in Fig. 2, the expression levels of proteins HSP90B1, PDIA6 and GSTP1 were significantly higher after radiation treatment. In the polysaccharides and flavones treatment groups, the expression levels of the three detected proteins were lower than those of the radiation controls. Protein HSP90B1 is a member of the Hsp90 family which is involved in maintaining protein homeostasis in the cell secretory pathway as well as functioning in the intracellular trafficking of peptides from the extracellular space to the MHC class I antigen processing pathway of antigen presentation cells. HSP90B1 plays a key role in signal transduction, protein folding and protein degradation. Protein PDIA6 belongs to the protein disulfide isomerase family and is found in the endoplasmic reticulum where it catalyzes the formation and breakage of disulfide bonds between cysteine residues within proteins as they fold. Protein GSTP1 plays a pivotal role in the detoxification of xenobiotics, as well as in carcinogenesis and drug resistance. Our data have demonstrated that polysaccharides and flavones extracted from LAS significantly repressed the expression changes of these detected proteins induced by heavy ion radiation. Not with standing any activities that may have been caused by impurities in the extracted samples, these results suggest that polysaccharides and flavones derived from LAS could significantly decrease radiation-induced damage in the livers of mice.
4. Conclusions
This study has indicated that polysaccharides and flavones extracted from wild Acanthopanax senticosus from the Lesser Khingan Mountain range in China, could be radioprotective agents against ion-radiation induced oxidative damage in animal tissues. These data provide a new perspective on human radiation protection and are potentially of relevance to the protection of humans in occupations involving specialist types of work where there is radiation exposure. Also in our study, we observed that three different kinds of proteins extracted from mice livers were down regulated after mice were administered (through diet), with extracts of the active components derived from Acanthopanax senticosus. Further studies will need to focus on identifying the precise mechanism of regulation of these proteins during the anti-radiation response.Author contributions
Weihong Lu conceived this study; Wei Song wrote the paper; Jinming Shi conducted the experiments; Denis Baranenko and Jing Jing analyzed the data.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research was supported by the Outstanding Academic Leaders Foundation of Harbin city (2013RFXXJ042), the Advanced Project for Aerospace (040102) and Planning Project for Space Application (01-1-08).References and Notes
- H. H. Smith, Comparative genetic effects of different physical mutagens in higher plants, in Induced mutations and plant improvement, International Atomic Energy Agency, Vienna, 1972, pp. 75–93 Search PubMed.
- N. Shikazono, Y. Yokota, S. Kitamura, C. Suzuki, H. Watanabe, S. Tano and A. Tanaka, Mutation rate and novel tt mutants of Arabidopsis thaliana induced by carbon ions, Genetics, 2003, 163, 1449–1455 CAS.
- S. J. Hosseinimehr, Trends in the development of radioprotective agents, Drug Discovery Today, 2007, 12, 794–805 CrossRef CAS PubMed.
- G. C. Jagetia, Radioprotective potential of plants and herbs against the effects of ionizing radiation, J. Clin. Biochem. Nutr., 2007, 40, 74–81 CrossRef CAS.
- X. J. Li and H. Y. Zhang, Western-medicine-validated anti-tumor agents and traditional Chinese medicine, Trends Mol. Med., 2008, 14, 1–2 CrossRef CAS.
- F. Wu, H. Li, L. Zhao, X. Li, J. You, Q. Jiang, S. Li, L. Jin and Y. Xu, Protective effects of aqueous extract from Acanthopanax senticosus against corticosterone-induced neurotoxicity in PC12 cells, J. Ethnopharmacol., 2013, 148, 861–868 CrossRef.
- C. C. Lin and P. C. Huang, Antioxidant and hepatoprotective effects of Acanthopanax senticosus, Phytother. Res., 2000, 14, 489–494 CrossRef CAS.
- S. H. Kim, S. Young Park, E. Kyoung Kim, E. Yeon Ryu, Y. Hun Kim, G. Park and S. Joon Lee, Acanthopanax senticosus has a heme oxygenase-1 signaling-dependent effect on Porphyromonas gingivalis lipopolysaccharide-stimulated macrophages, J. Ethnopharmacol., 2012, 142, 819–828 CrossRef PubMed.
- E. J. Lim, G. M. Do, J. H. Shin and O. Kwon, Protective effects of Acanthopanax divaricatus vat. albeofructus and its active compound on ischemia-reperfusion injury of rat liver, Biochem. Biophys. Res. Commun., 2013, 432, 599–605 CrossRef CAS PubMed.
- X. L. Li and A. G. Zhou, Preparation of polysaccharides from Acanthopanax senticosus and its inhibition against irradiation-induced injury of rat, Carbohydr. Polym., 2007, 67, 219–226 CrossRef CAS.
- J. Fang, F. Y. Yan, X. F. Kong, Z. Ruan, Z. Q. Liu, R. L. Huang, T. J. Li, M. M. Geng, F. Yang, Y. Z. Zhang, P. Li, J. H. Gong, G. Y. Wu, M. Z. Fan, Y. L. Liu, Y. Q. Hou and Y. L. Yin, Dietary supplementation with Acanthopanax senticosus extract enhances gut health in weanling piglets, Livest. Prod. Sci., 2009, 123, 268–275 CrossRef.
- W. Li, Q. Luo and L. H. Jin, Acanthopanax senticosus extracts have a protective effect on Drosophila gut immunity, J. Ethnopharmacol., 2013, 146, 257–263 CrossRef CAS PubMed.
- X. Wang, C. X. Hai, X. Liang, S. X. Yu, W. Zhang and Y. L. Li, The protective effects of Acanthopanax senticosus harms aqueous extracts against oxidative stress: role of Nrf2 and antioxidant enzymes, J. Ethnopharmacol., 2010, 127, 424–432 CrossRef CAS.
- H. H. Sun, W. J. Mao, Y. Chen, S. D. Guo, H. Y. Li, X. H. Qi, Y. L. Chen and J. Xu, Isolation, chemical characteristics and antioxidant properties of the polysaccharides from marine fungus Penicillium sp. F23-2, Carbohydr. Polym., 2009, 78, 117–124 CrossRef CAS.
- M. Q. Hui, T. Z. Ting and B. Z. Quan, Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (chlorophyta), J. Appl. Phycol., 2005, 17, 527–534 CrossRef.
- S. M. Liu, X. Z. Li, Y. Huo and F. Lu, Protective effect of extract of Acanthopanax senticosus harms on dopaminergic neurons in Parkinson's disease mice, Phytomedicine, 2012, 19, 631–638 CrossRef CAS.
- Z. F. Zhang, Y. Liu, P. Luo and H. Zhang, Separation and purification of two flavone glucuronides from Erigeron multiradiatus (Lindl.) Benth with macroporous resins, J. Biomed. Biotechnol., 2009, 1, 83–99 Search PubMed.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. A. Rebers and F. Smith, Colorimetric method for determination of sugars and related substances, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- A. Floegel, D. O. Kim, S. J. Chung, W. O. Song, M. L. Fernandez, R. S. Bruno, S. I. Koo and O. K. Chun, Development and validation of an algorithm to establish a total antioxidant capacity database of the US diet. Int, J. Food Sci. Nutr., 2010, 61, 600–623 CAS.
- S. M. Liu, X. Z. Li, Y. Huo and F. Lu, Protective effect of extract of Acanthopanax senticosus Harms on dopaminergic neurons in Parkinson's disease mice, Phytomedicine, 2012, 19, 631–638 CrossRef CAS PubMed.
- Y. Yin, F. Y. Gong, X. X. Wu, Y. Sun, Y. H. Li, T. Chen and Q. Xu, Anti-inflammatory and immunosuppressive effect of flavones isolated from Artemisia vestita, J. Ethnopharmacol., 2008, 120, 1–6 CrossRef CAS PubMed.
- W. Brand-Williams, M. E. Cuvelier and C. Berset, Use of a free radical method to evaluate antioxidant activity LWT, Food Sci. Technol., 1995, 28, 25–30 CAS.
- G. Sanchez, E. Jimenez and C. F. Saura, In vitro antioxidant activity of coffees brewed using different procedures (Italian, espresso and filter), Food Chem., 2005, 90, 133–140 CrossRef.
- H. Ohkawa, N. Ohishi and Y. Yagi, Assay of lipid peroxides in animal tissue by thiobarbituric acid reaction, Anal. Biochem., 1979, 95, 351–358 CrossRef CAS.
- C. H. Beuchamp and I. Fridovich, Superoxide dismutase: improved assays and an assay applicable to acrylamide gels, Anal. Biochem., 1971, 44, 276–287 CrossRef.
- J. M. McCord and I. Fridovich, Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein), J. Biol. Chem., 1969, 244, 6049–6055 CAS.
- M. M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS.
- N. R. Kitteringham, A. Abdullah, J. Walsh, L. Randle, R. E. Jenkins, R. Sison, C. E. Goldring, H. Powell, S. C. Anderson, S. Williams, L. Higgins, M. Yamamoto, J. Hayes and B. K. Park, Proteomic analysis of Nrf2 deficient transgenic mice reveals cellular defence and lipid metabolism as primary Nrf2-dependent pathways in the liver, J. Proteomics, 2010, 73, 1612–1631 CrossRef CAS.
- A. Gorg, C. Obermaier, G. Boguth, A. Harder, B. Scheibe, R. Wildgruber and W. Weiss, The current state of two-dimensional electrophoresis with immobilized pH gradients, Electrophoresis, 2000, 21, 1037–1053 CrossRef CAS.
- M. Fountoulakis, P. Berndt, U. A. Boelsterli, F. Crameri, M. Winter, S. Albertini and L. Suter, Two-dimensional database of mouse liver proteins: changes in hepatic protein levels following treatment with acetaminophen or its nontoxic regioisomer 3-acetamidophenol, Electrophoresis, 2000, 21, 2148–2161 CrossRef CAS.
- K. Thaipong, U. Boonprakob, K. Crosby, L. Cisneros-Zevallos and D. Hawkins Byrne, Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts, J. Food Compos. Anal., 2006, 19, 669–675 CrossRef CAS.
- A. Floegel, D. O. Kim, S. J. Chung, S. I. Koo and O. K. Chun, Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods, J. Food Compos. Anal., 2011, 24, 1043–1048 CrossRef CAS.
- H. Cheng, S. Feng, X. Jia, Q. Li, Y. Zhou and C. Ding, Structural characterization and antioxidant activities of polysaccharides extracted from Epimedium acuminatum, Carbohydr. Polym., 2013, 92, 63–68 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |