Removal of ciprofloxacin from aqueous solution using long TiO2 nanotubes with a high specific surface area†
Kai Zhenga,
Xingye Zhenga,
Fei Yu*c and
Jie Ma*b
aDepartment of Environmental Engineering, Nanjing Institute of Technology, Nanjing, 211167, P. R. of China
bState Key Laboratory of Pollution Control and Resource Reuse, School of Environmental Science and Engineering, Tongji University, 1239 Siping Road, Shanghai 200092, P. R. of China. E-mail: fyu@vip.163.com; Tel: +86 21 6598 1831
cCollege of Chemistry and Environmental Engineering, Shanghai Institute of Technology, Shanghai 2001418, P. R. of China. E-mail: jma@tongji.edu.cn; Tel: +86 21 6087 3182
First published on 7th December 2015
Abstract
Long TiO2 nanotubes (TNs) were successfully prepared by the reaction of TiO2 and NaOH. The raw materials were treated by stirring, ion exchange, centrifugation, and freeze-drying, and then the target TNs was synthesized. Anatase TNs were obtained by calcinating the TNs at 823 K for 4.5 h. The TNs were characterized by Brunauer–Emmett–Teller surface area analysis, X-ray diffraction analysis, scanning electron microscopy, transmission electron microscopy, and X-ray photoelectron spectrometry. The results indicated that the TNs had a larger specific surface area (ca. 160 m2 g−1) and pore volume (ca. 0.6 cm3 g−1) than the commercial product P25. The adsorption of ciprofloxacin onto the TNs was compared with their adsorption onto P25. The adsorption isotherm, kinetics, and regeneration performance were investigated. The experimental results indicated that the maximum adsorption capacity of the TNs and P25 was 26.38 and 5.32 mg g−1, respectively, and their adsorption behavior was better fitted by the Langmuir model than by the Freundlich model. The kinetic regression results showed that the adsorption kinetics were more accurately represented by a pseudo-second-order model than by a pseudo-first-order model; the rate of the pseudo-second-order reactions on P25 and the anatase TNs were 0.0442 and 0.27463 min−1, respectively. After adsorption, the TNs had better regeneration properties than P25 under UV irradiation at 500 W for 3 h in 5 mL of aqueous solution. These results show that long TNs have a better adsorption capacity and regeneration properties than P25. This study provides a green method for the removal of organic pollutants by combining enrichment by adsorption with photocatalytic degradation.
1. Introduction
Ciprofloxacin (CIP) is a broad-spectrum quinolone antibiotic and is widely used in human and veterinary medicine.1 CIP is added to poultry feed to guard against disease during the growth period; however, the fluorinated functional groups in CIP are not completely metabolized.2,3 More than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of antibiotics were used in Europe in 1999, with farm animals and therapeutic drugs accounting for 35 and 29%, respectively; this represents a decrease of 50% since 1997. On release into the environment, CIP is difficult to degrade4 and has been detected in surface waters and effluent-dominated systems in the USA,5 Canada,6–8 and Europe at concentrations from ng L−1 to μg L−1.9–12
000 tons of antibiotics were used in Europe in 1999, with farm animals and therapeutic drugs accounting for 35 and 29%, respectively; this represents a decrease of 50% since 1997. On release into the environment, CIP is difficult to degrade4 and has been detected in surface waters and effluent-dominated systems in the USA,5 Canada,6–8 and Europe at concentrations from ng L−1 to μg L−1.9–12
The detection of CIP in the atmosphere, water and soil presents some health concerns. The discharge of wastewater containing CIP into aquatic environments may lead to antibiotic resistance within native bacterial populations. Fluoroquinolones are one of the most important types of antibiotic with respect to annual sales and the variability of the drugs.13,14 They are effective in treating infections caused by all kinds of bacteria and are regarded as the antibiotic of last resort when other treatments are ineffective. CIP is a fluoroquinolone derivative and is a principal metabolite of enrofloxacin. CIP is present in different forms depending on the solution pH. The main species of CIP include negative ions, positive ions, and amphoteric ions. The form of CIP can affect many of its properties, such as adsorption in soils, photolysis, and its activity in the target species.15–17
Adsorption and photocatalytic technology have been widely utilized to remove CIP from aqueous solutions. One of the advantages of adsorption is its low cost. However, the adsorption process is incomplete as a result of the heterogeneity of the adsorbents and the difficulty in completely decomposing them. Adsorbed CIP has been widely found in treated water bodies.18 CIP on adsorbents applied to the land is not efficiently decomposed and may later be released into water bodies. As adsorbents have a finite capacity for pollutant molecules, it is necessary either to regenerate or dispose of the adsorbents after use.
Organic pollutants can be removed or destroyed by photocatalytic oxidation. The photocatalytic activity depends on the ability of the catalyst to create electron–hole pairs, which generate free radicals (e.g. hydroxyl radicals, ˙OH) that undergo secondary reactions. However, photocatalytic oxidation has not been widely applied in practical wastewater treatment. The removal of pollutants by the photocatalytic oxidation of TiO2 has the disadvantage that the existing particles and chrominance from the pollutants may cause the refraction, reflection and scattering of light. In addition, the rate of catalysis of the photocatalyst is much greater than the adsorption and diffusion speed, which may lead to further electron–hole pair recombination during photocatalysis. Traditional TiO2 photocatalysts and existing photocatalytic technology have a low catalytic efficiency, high energy consumption, and high operational costs.19 It is still challenging to extend the application of photocatalytic oxidation technology to the removal of organic pollutants.
We divided traditional adsorption–catalysis into two separate processes. The pollutants were first enriched on the adsorbent and were then placed in clear water to catalyze after preferential adsorption. This concentrated the pollutants and facilitated their mineralization so that the catalyst or adsorbents were regenerated and the contaminants were removed via mineralization.
Many synthetic routes for the various morphologies and crystallite phases of TiO2 have been reported previously. Among the most commonly used methods are sol–gel methods,20 hydrothermal reactions,21 solvothermal reactions,22 anodic oxidation,23 the use of a hard template,24 and direct oxidation.25 Titanium dioxide has been used for the degradation of CIP via its photocatalysis properties under UV irradiation.26 However, the surface area and pore volume of commercial TiO2 are only about 50 m2 g−1 and 0.1 cm3 g−1, respectively. This low surface area and pore volume restrict its adsorption and limit its performance as a catalyst. To overcome these shortcomings, the structure of TiO2 needs to be improved to enhance its adsorption capacity and catalyzing efficiency.
The use of TiO2 nanotubes improves the catalytic performance. The most commonly used preparation methods are: crystallization of amorphous anodized TiO2 nanotubes at low temperature;27 electrochemical etching and hydrothermal synthesis;28 and the treatment of TiO2 particles with NaOH.29 However, these procedures are time-consuming, complicated, and expensive and are not viable on an industrial scale. A cost-effective, large-scale preparation method for a stable, high surface area, mesoporous form of TiO2 still needs to be developed.
We report here the synthesis of long TiO2 nanotubes (TNs) with a high surface area (160 m2 g−1) using a hydrothermal method with stirring. The mechanical force-driven stirring process synchronously improved the diffusion and surface reaction rate of titanate nanocrystal growth in the solution phase, producing long TNs. The TNs had outstanding adsorption as a result of their larger specific surface area (SSA) and could be used to efficiently adsorb and remove pollutants. The TNs also had an excellent catalyzing performance and could be used to both mineralize pollutants and regenerate the adsorbent. This method provides an effective approach for the efficient removal of pollutants from contaminated waters.
2. Experimental
2.1 Materials and chemicals
All chemicals were of analytical-reagent grade and were purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China) and used without further purification. All solutions were prepared using deionized water.2.2 Preparation of TiO2 and TNs
TiCl4 (25 g, purity 99%) was added to 2 L of deionized water with 2 mL of concentrated sulfuric acid. After the TiCl4 had been completely hydrolyzed, 120–140 mL of 17% ammonia were added dropwise to give a pH between 7.0 and 7.5. The raw mixture was filtered and rinsed with deionized water to remove NH4Cl until the leaching liquid did not produce a white precipitate with 0.2 mmol L−1 Ag2SO4. The filtered residue was successively desiccated at 373 K for 2 h and vacuum-dried at 353 K at a pressure of 10−1 Pa for 3 h to give Ti(OH)4. The anatase crystal form of TiO2 was obtained by heating Ti(OH)4 at 823 K for 4.5 h.A 3 g mass of anatase titanium dioxide was added to 350 mL of 10 mol L−1 NaOH solution and the mixture was stirred ultrasonically for 2 h and then magnetically at 200 rpm for 2 h. The solution and a magnetic rotator were then placed in a 500 mL Teflon reactor. The reactor was placed into a heater with a magnetic stirring apparatus (RCT Basic, IKA Corporation, Germany). The reactor was operated at about 600 rpm at 403 K for 20–24 h.
Sodium titanate was obtained by centrifugation and then rinsed five times until the pH of washing solution was 11. Hydrochloric acid (0.5 mol L−1) was then added in batches to adjust the pH of the solution to 2; this was maintained for 3–4 h to exchange hydrogen ions and with the sodium ions in solution. The mixture was then rinsed five times until the pH of the washing solution was 6.8–7.0 and it was then filtered to give the hydrogen titanate nanotubes. The residue was dried under vacuum. Crystals of long TNs were obtained at 823 K after 4.5 h.
2.3 Batch adsorption experiments
The concentration of CIP was determined by colorimetry at the maximum absorbance (λmax = 275 nm). A calibration graph was plotted to obtain the absorbance–concentration profile of CIP. At high concentrations of CIP, the dye samples were diluted before the absorbance measurements. The concentration of the CIP in the solution was determined by the Beer–Lambert law.30 Batch adsorption experiments were conducted in 50 mL glass bottles with 20 mg of the adsorbent and 40 mL of a CIP solution with different initial concentrations of 5–50 mg L−1. The pH of the solution was adjusted to ca. 7.0 with HCl or NaOH solutions. The timing of the adsorption period started as soon as the solution was poured into the bottle. Sample bottles were agitated on a shaker (TS-2102C, Shanghai Tensuclab Instruments Manufacturing Co. Ltd, China) operated at a constant temperature of 25 °C and 150 rpm for 24 h to achieve adsorption equilibrium. All adsorption experiments were conducted in duplicate and only the mean values are reported. The maximum deviation for the duplicates was usually <5%. After adsorption equilibrium had been achieved, the CIP concentrations of the solutions were measured using a spectrophotometer (UV759UV-VIS, Shanghai Precision & Scientific Instrument Co. Ltd). Kinetic studies were performed at a constant temperature of 25 °C and 150 rpm with 25 (P25) and 50 (TN) mg L−1 initial concentration of the CIP solutions.The amount of CIP adsorbed (qt mg g−1) was calculated as:
 | (1) |
2.4 Regeneration studies
After adsorption, the TN and P25 adsorbents were placed in a quartz pot in a black box under UV irradiation from a 500 W ultraviolet lamp for 3 h. The samples were rinsed with distilled water and then dehydrated by freeze-drying. The adsorption experiments were conducted on the adsorbent from the first regeneration cycle. The process was repeated six times to study the ability of the photocatalytic technology to regenerate the adsorbents.2.5 Characterization
The surface morphologies of the samples were characterized using field-emission scanning electron microscopy (FE-SEM, Hitachi S-4800) operating at a typical accelerating voltage of 10 kV. The microstructure and morphology of the samples were analyzed using high-resolution transmission electron microscopy (TEM, JEOL JEM 2100F, accelerating voltage 200 kV). To determine the existing state of atoms, X-ray photoelectron spectroscopy (XPS) was carried out on a Kratos Axis Ultra DLD spectrometer using monochromatic Al Kα X-rays at a pressure of 1 × 10−9 Torr. A Unico UV2100 spectrophotometer was used to determine the CIP concentration at a wavelength of 275 nm.3. Results and discussion
3.1 Characterization of TNs
Fig. 1 shows that the TiO2 powder was transformed into elongated nanotubes. During the reaction, the TiO2 dissolved in the NaOH solution was converted into sodium titanate. Saturated sodium titanate can form crystals. The saturated sodium titanate was vigorously stirred, which produced both centrifugal and shearing forces; the centrifugal force and the stirring speed had linear relationship, whereas the shearing force and stirring speed had a curved relationship. The diameter of the TNs was closely related to the centrifugal force and the length of the TNs was closely related to the shearing force. Thus the structure of the TNs can be effectively controlled by optimizing the experimental parameters (temperature, time, mixing speed, post-treatment of raw materials). It can be seen from Fig. 1e and f that the length of prepared anatase TNs ranged from 1.0 to 2 μm, which is longer than that in previous reports (Table S1†) and the TNs had the shape of a hollow tube.Fig. 2a shows that the prepared TNs gave a single strong peak at maximum diffraction angles of 25.3 and 48.2°; triple peaks were seen at 36.9, 37.8 and 38.61° and a medium double peaks at 54 and 55.3°. The characteristics of the TNs were almost the same as those of standard anatase TiO2 (PDF#00-002-0387). These results further indicated that the prepared anatase TNs had the form of anatase crystals. Fig. S1† shows that sodium titanate exhibited strong peaks attributed to titanate at about 10°. The results showed that peaks at about 10° were weaker when the sodium titanate had been exchanged with hydrogen ions, confirming that sodium titanate had been converted into titanium dioxide. The results from Fig. S1† indicate that the crystalline form of the TN heated at 823 K for 4.5 h was anatase. The experimental results from Fig. 2b and c show that the product had a maximum SSA (ca. 164 m2 g−1) and pore volume (ca. 0.598 cm3 g−1) when the mixing speed was maintained at 500 rpm at 403 K for 18 h.
 | ||
| Fig. 2 (a) XRD patterns, (b) N2 adsorption and desorption isotherms, and (c) distribution of pore diameters of the TNs. | ||
As can be seen from Fig. 3b and c, the binding energy of O1s was 529.5 eV and the binding energies of Ti2p3 were 458.5 and 464.5 eV, identical to those reported previously,31,32 and indicating that TNs retained the same structure as the TiO2.
3.2 Adsorption isotherms
The isotherms in Fig. 4 show that the adsorption capacities of the TNs and P25 were 26.38 and 5.32 mg g−1, respectively. The higher adsorption capacity of the TNs was a result of the higher SSA and pore volume (Fig. 2c) (ca. 50 m2 g−1 and 0.1 cm3 g−1, respectively). The fitted curves in Fig. 4 show that the Langmuir model fitted the adsorption behavior better than the Freundlich model. Table 1 gives R2 values for the Langmuir model of CIP adsorption onto P25 and TN as 0.97735 and 0.94364, respectively. This was attributed to fewer functional groups in the structures of P25 and TN. Their adsorption was mainly a result of physical adsorption onto the monomolecular layer.333.3 Adsorption kinetics of CIP
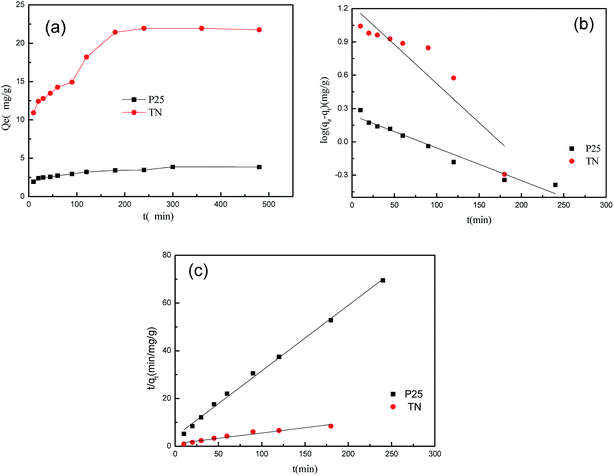
The equilibrium times for the adsorption of CIP onto P25 and TN are shown in Fig. 5a and were 300 and 200 min, respectively. The physical and chemical effects of all the adsorption processes involve the mass transfer of a substance from the liquid phase to the adsorbent surface to form a complex between CIP and the adsorbent. CIP solutions with an initial concentration of 50 mg L−1 were used to investigate the adsorption kinetics of CIP on the TNs. The adsorption of CIP onto the TNs was rapid and reached equilibrium in ca. 180 min. The concentration reduced stepwise due to the reduction of adsorption sites on the TNs during the adsorption process. However, the removal of CIP in solution by adsorption on P25 was slow and required ca. 300 min to reach equilibrium. A longer equilibrium time was required for the adsorption of CIP onto P25 than onto the TNs, which may be attributed to the different pore structures of the TNs and P25.Pseudo-first-order and pseudo-second-order kinetic models34,35 were used to further investigate the CIP adsorption process and the kinetic parameters are given in Table 2. The adsorption of CIP onto TN and P25 were better fitted with the pseudo-second-order model based on the correlation coefficient (R2). The k2 value for the adsorption of CIP onto the TNs was larger than the value for P25, which showed that it was adsorbed faster onto the TNs than onto P25. The calculated q values (qe,cal) derived from the pseudo-second model were more reliable than those from the pseudo-first-order model.
| Adsorbent | Initial conc. (mg L−1) | qe,exp (mg g−1) | Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|---|---|---|
| k1 (min−1) | qe,cal (mg g−1) | R2 | k2 (min−1) | qe,cal (mg g−1) | R2 | |||
| a CIP concentration = 10 or 25 mg L−1; TN or P25 = 0.5 g L−1. CIP concentration = 10 or 25 mg L−1; TN or P25 = 0.5 g L−1. | ||||||||
| P25 | 5.0 | 26.28 | 0.0029 | 4.14 | 0.9489 | 0.0442 | 4.14 | 0.9965 |
| TN | 25 | 5.32 | 0.0070 | 22.2 | 0.8288 | 0.2746 | 22.2 | 0.9473 |
As shown in Fig. 5a, CIP was adsorbed faster and to a greater extent on the TNs than on P25, which was attributed to the larger SSA and pore volume of the TNs (Fig. 2b). The mesoporous character of the TNs allowed CIP to diffuse into the pore canals of the TNs at a faster speed. The mesoporous characteristics of TN are helpful in enhancing the photocatalytic process.
3.4 Regeneration properties of TN and P25
P25 and TN had good regeneration capacities under UV irradiation with a 500 W ultraviolet lamp for 3 h in 5 mL of aqueous solution, as shown in Fig. 6. This self-regeneration process could be recycled more than six times with no decrease in adsorption capacity. The saturated sorption capacity was ca. 16 mg g−1 after the sixth cycle of regeneration with a UV lamp, indicating that the effectiveness of the TN adsorbent did not significantly change from the first to the sixth cycle.4. Conclusions
Long TNs were successfully prepared by the reaction of TiO2 and NaOH by a hydrothermal method with stirring. The TNs showed outstanding adsorption as a result of their large SSA (ca. 160 m2 g−1) and pore volume (ca. 0.6 cm3 g−1), which was larger than that of commercial P25, and were used to efficiently adsorb and remove CIP from aqueous solution. The experimental results indicated that the TNs have excellent adsorption of 26.38 mg g−1. The TNs also had better regeneration properties after the adsorption and mineralization of pollutants. This method provides a green route for the removal of organic pollutants by combining adsorption enrichment with photocatalytic degradation.Acknowledgements
This research was supported by the Fundamental Research Funds for the Central Universities and Undergraduate Innovation Fund Project (N20151201). We are also grateful to the anonymous reviewers for their valuable comments to improve this manuscript.References
- S. Martin, A. Shchukarev, K. Hanna and J. F. Boily, Environ. Sci. Technol., 2015, 49, 12197–12205 CrossRef CAS PubMed.
- T. Paul, P. L. Miller and T. J. Strathmann, Environ. Sci. Technol., 2007, 41, 4720–4727 CrossRef CAS PubMed.
- Y. L. Liao, W. X. Que, P. Zhong, J. Zhang and Y. C. He, ACS Appl. Mater. Interfaces, 2011, 3, 2800–2804 CAS.
- C. G. Daughton, Lancet, 2002, 360, 1035–1036 CrossRef.
- D. W. Kolpin, E. T. Furlong, M. T. Meyer, E. M. Thurman, S. D. Zaugg, L. B. Barber and H. T. Buxton, Environ. Sci. Technol., 2002, 36, 4004 CrossRef CAS.
- C. Y. Hao, L. Lissemore, B. Nguyen, S. Kleywegt, P. Yang and K. Solomon, Anal. Bioanal. Chem., 2006, 384, 505–513 CrossRef CAS PubMed.
- L. Lissemore, C. Hao, P. Yang, P. K. Sibley, S. Mabury and K. R. Solomon, Chemosphere, 2006, 64, 717–729 CrossRef CAS PubMed.
- C. D. Metcalfe, B. G. Koenig, D. T. Bennie, M. Servos, T. A. Ternes and R. Hirsch, Environ. Toxicol. Chem., 2003, 22, 2872–2880 CrossRef CAS PubMed.
- B. Halling-Sorensen, S. N. Nielsen, P. F. Lanzky, F. Ingerslev, H. C. H. Lutzhoft and S. E. Jorgensen, Chemosphere, 1998, 36, 357–394 CrossRef CAS PubMed.
- T. Heberer, Toxicol. Lett., 2002, 131, 5–17 CrossRef CAS PubMed.
- O. A. H. Jones, N. Voulvoulis and J. N. Lester, Environ. Technol., 2001, 22, 1383–1394 CrossRef CAS PubMed.
- K. Kümmerer, Chemosphere, 2001, 45, 957–969 CrossRef.
- M. C. Dodd, A. D. Shah, U. von Gunten and C. H. Huang, Environ. Sci. Technol., 2005, 39, 7065–7076 CrossRef CAS PubMed.
- Y. Picó and V. Andreu, Anal. Bioanal. Chem., 2007, 387, 1287–1299 CrossRef PubMed.
- C. Gu and K. Karthikeyan, Environ. Sci. Technol., 2005, 39, 9166–9173 CrossRef CAS PubMed.
- M. C. Dodd, M. O. Buffle and U. von Gunten, Environ. Sci. Technol., 2006, 40, 1969–1977 CrossRef CAS PubMed.
- K. Torniainen, S. Tammilehto and V. Ulvi, Int. J. Pharm., 1996, 132, 53–61 CrossRef CAS.
- R. Andreozzi, V. Caprio, C. Ciniglia, M. de Champdore, R. Lo Giudice, R. Marotta and E. Zuccato, Environ. Sci. Technol., 2004, 38, 6832–6838 CrossRef CAS PubMed.
- T. Paul, P. L. Miller and T. J. Strathmann, Environ. Sci. Technol., 2007, 41, 4720–4727 CrossRef CAS PubMed.
- Y. M. Sun, J. H. Seo, C. J. Takacs, J. Seifter and A. J. Heeger, Adv. Mater., 2011, 23, 1679–1683 CrossRef CAS PubMed.
- C. W. Cheng, B. Liu, H. Y. Yang, W. W. Zhou, L. Sun, R. Chen, S. F. Yu, J. X. Zhang, H. Gong, H. D. Sun and H. J. Fan, ACS Nano, 2009, 3, 3069–3076 CrossRef CAS PubMed.
- H. K. Yang, B. K. Moon, B. C. Choi, J. H. Jeong and K. H. Kim, J. Nanosci. Nanotechnol., 2013, 13, 6060–6063 CrossRef CAS PubMed.
- L. Yang, M. Viste, J. Hossick-Schott and B. W. Sheldon, Electrochim. Acta, 2012, 81, 90–97 CrossRef CAS.
- L. Y. Gong, X. H. Liu and L. H. Lu, Mater. Lett., 2012, 67, 226–228 CrossRef CAS.
- K. F. Huo, X. M. Zhang, J. J. Fu, G. X. Qian, Y. C. Xin, B. Q. Zhu, H. W. Ni and P. K. Chu, J. Nanosci. Nanotechnol., 2009, 9, 3341–3346 CrossRef CAS PubMed.
- X. H. Tang and D. Y. Li, Langmuir, 2011, 27, 1218–1223 CrossRef CAS PubMed.
- Y. L. Liao, W. X. Que, P. Zhong, J. Zhang and Y. C. He, ACS Appl. Mater. Interfaces, 2011, 3, 2800–2804 CAS.
- H. Kim, K. Noh, C. Choi, J. Khamwannah, D. Villwock and S. Jin, Langmuir, 2011, 27, 10191–10196 CrossRef CAS PubMed.
- T. Gao and B. P. Jelle, J. Phys. Chem. C, 2013, 117, 1401–1408 CAS.
- M. A. Behnajady, N. Modirshahla and H. Fathi, J. Hazard. Mater., 2006, 136, 816–821 CrossRef CAS PubMed.
- S. Sodergren, H. Siegbahn, H. Rensmo, H. Lindstrom, A. Hagfeldt and S. E. Lindquist, J. Phys. Chem. B, 1997, 101, 3087–3090 CrossRef.
- B. Erdem, R. A. Hunsicker, G. W. Simmons, E. D. Sudol, V. L. Dimonie and M. S. El-Aasser, Langmuir, 2001, 17, 2664–2669 CrossRef CAS.
- J. Ma, F. Yu, L. Zhou, L. Jin, M. X. Yang, J. S. Luan, Y. H. Tang, H. B. Fan, Z. W. Yuan and J. H. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 5749–5760 CAS.
- R. M. Tinnacher, P. S. Nico, J. A. Davis and B. D. Honeyman, Environ. Sci. Technol., 2013, 47, 6214–6222 CAS.
- P. F. Salipante and S. D. Hudson, Langmuir, 2015, 31, 3368–3376 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra17956d |
| This journal is © The Royal Society of Chemistry 2016 |