Design of folic acid conjugated chitosan nano-cur–bioenhancers to attenuate the hormone-refractory metastatic prostate carcinoma by augmenting oral bioavailability†
Monika Sharmaac,
Shweta Sharmab,
Vikas Sharmab,
Satish Agarwalb,
Pankaj Dwivedib,
Sarvesh K. Paliwalc,
Jagdamba Prasad Maikurib,
Anil K. Dwivedib,
Gopal Guptab,
Prabhat R. Mishrab and
A. K. S. Rawat*a
aPharmacognosy and Ethnopharmacology Division, CSIR-National Botanical Research Institute, Lucknow, U.P, India-226001. E-mail: pharmacognosy1@gmail.com
bPharmaceutics & Endocrinology Divisions, CSIR-Central Drug Research Institute, Lucknow, U.P, India-226031
cPharmaceutics Department, Banasthali Vidyapeeth, Banasthali, Rajasthan, India-304022
First published on 5th February 2016
Abstract
The objective of this study is to develop a folic acid (FA) functionalized co-delivery system for oral delivery of curcumin (CUR) with bioenhancers (B) (“trikatu”) to attenuate hormone refractory prostate cancer. The nano-cur–bioenhancer exhibited significantly elevated cytotoxicity in a prostate cancer cell line (PC3) compared to CUR. Further, mechanistic studies in PC3 cells revealed that nano-cur–bioenhancers induce apoptosis by mitochondrial membrane disruption and a ROS mediated pathway. Collaborative efforts of bioenhancers, mucoadhesive chitosan (CH) and active uptake through FA governed the success of the nanoparticles (NPs) as evidenced through the cell uptake experiments and Hoechst assay for P-glycoprotein functioning. Pharmacokinetic results confirmed nearly 7.7 fold increases in Cmax of the nano-cur–bioenhancer CUR–B–CH-NPs as compared to a CUR solution and the AUC increased nearly 6 fold in CUR–B–CH-NPs than CUR. Thus, escalating drug uptake in prostate cancer PC3 and Caco-2 cell monolayers along with enhanced bioavailability substantiated effective oral delivery of nano-cur–bioenhancers. Thus, nano-cur–bioenhancers have remarkable potential as a multifunctional oral delivery system for management of hormone refractory prostate cancer.
1. Introduction
In recent years, prostate cancer has emerged one of the most common and fatal male malignancies. Current therapeutic options available for management of prostate cancer are surgery, radiation and adjuvant hormonal therapy which are strictly as per the stage of prostate cancer. The success stories of these therapies are limited only to the early stages with localized prostate cancer but in advanced stages such as stage IV hormonal treatment is the preferred therapy. However in recent times, resistance to hormone therapy has been observed which led to the development of androgen independent hormone refractory prostate cancer. Though chemotherapy has emerged as most regular approach for androgen independent prostate cancer but it has its pros and cons.1,2 The chemotherapy has its own restriction mainly by two important factors: multidrug resistance (MDR) and toxicity.3,4 MDR is most commonly due to the over-expression of P-glycoprotein (P-gp) in the plasma membrane of cancer cells causing drug efflux mechanism.5,6 This efflux often leads to reduced drug–cell concentration due to lower accumulation inside the cancer cells and results in toxic effects. Different approaches were materialized to overcome MDR effect caused by P-gp barrier including co-administration of P-gp inhibitors with the anticancer drug. While, P-gp is expressed abundantly in cancer cells but is also available in normal cells, such as kidney, liver, adrenal glands, blood brain barrier and gastrointestinal track.7,8 Expected non-selective binding can be possible with P-gp inhibitors to normal tissues facilitating undesirable pharmacokinetic profiles and toxicity.9,10This selectivity issue can be eradicated using alternative approach of NPs as drug delivery vehicles that can be exploited to bypass P-gp mediated efflux mechanism on tumour cell membrane, by “P-gp bypassing effect”.11,12 P-gp bypassing effect is a characteristic of NPs and is assessed by their direct uptake by the cells. This active uptake of NPs could also be possible due to various endocytic processes exploited by enterocytes such as macropinocytosis, clathrin-mediated endocytosis, caveolae-mediated endocytosis and clathrin- and caveolae-independent endocytosis. Amongst these, caveolae mediated endocytosis most effectively participates in transcellular transport of NPs.13 Consequently, polymeric carriers conjugated to FA have been reported to facilitates folate receptor mediated endocytosis by folate receptors (FRs) and thus, enhance drug internalization into cells. FA conjugated polymeric system, binds with FRs which is present abundantly on cancer cells, causes drug to accumulate selectively in cancer cells.14 These together can bring forward a working phenomenon for reducing the off-target side effects related with the chemotherapeutics.
CH is a mucoadhesive natural polysaccharide which originates from chitin of shrimp/crabs. Due to its mucoadhesive properties CH-NPs have the desired characters corresponding to nano-range size, better retention time and sustained release of drug for oral delivery. This protects anticancer drugs from acidic degradation in the gastrointestinal fluids and helps them to bypass the first-pass liver and intestinal metabolism.15 CH-NPs also circumvent drug extrusion through P-gp efflux pump etc. Previous studies have established CH as an ideal carrier of phyto-compounds, e.g. CH-NPs of epigallocatechin gallate have shown significant improvement of prostate cancer tumors.16 Jing et al., designed folic acid modified water soluble chitosan derivatives by chemical modifications for folate receptor mediated targeting. Further studies on the folic acid modified chitosan labelled with 99Tc explained the ability of chitosan backbone for designing folate specific targeting agents.17,18 CH is a natural polymer with attractive biodegradable and biocompatible attributes. Moreover its use in oral delivery is well known. Therefore we developed FA conjugated CH-NPs for the oral delivery of CUR with bioenhancers. The method of preparation was ionic gelation assisted by electrostatic interaction induced on addition of TPP in CH solution to form nanoparticles.19,20 Being a highly complex composite, it is extremely unstable in acidic environment, hence; it became very important to resolve this issue for oral delivery of bioenhancer phyto formula. CH nanostructures are potential carriers of phytocompounds, this feature had gained our interest in developing CH as phytocarrier encapsulating phytoformula. Also, CH improves stability, bioavailability and cellular uptake of CUR.21
CUR is a polyphenolic molecule which is extracted from the rhizome of the plant Curcuma longa and is a promising candidate for its anticancer potential. Several reports have revealed that CUR possesses anti-inflammatory, anti-oxidant, anti-proliferative, and anti-angiogenic properties against several cancer cell types and also demonstrates anti-microbial activities.22 The anticancer property of CUR specifically against prostate cancer cells (LNCaP) has been confirmed in vivo by the observation of the growth of tumors implanted in nude mice.23 CUR is also under clinical trials specifically for cancer and related diseases but its potential is hampered due its poor water solubility and short biological half-life which results in low oral bioavailability in plasma and tissue.24 For such complicated drugs co-delivery with natural bioenhancers is a proactive approach for successful delivery of drug having low bioavailability. Natural bioenhancers are chemical entities that augment the bioavailability of the drugs on mixing with them with or without any synergistic effect. The bioenhancers were also used in ayurvedic medicines as “trikatu”, which is equated to three herbs commonly used as spice i.e. black pepper (Piper nigrum Linn.), long pepper (Piper longum Linn.) and ginger (Zingiber officinale Rosc.) with active constituent piperine.25–27 Exploiting natural bio-enhancers with known anticancer drugs in delivery system apprehend enhanced bioefficacy and bioavailability with sustained action, enhanced surface area, rescue bioenhancers from degradation and target specificity. Further in current scenario active NPs uptake by enterocytes is also being exploited for delivery of NPs. Roger et al. report the development of folic acid functionalized PLGA-NPs for oral delivery of paclitaxel and where folic acid conjugation result in 3 fold improvement of absorption as compare to plain PLGA-NPs.13 Here also we report the use of folic acid conjugate CH-NPs for oral delivery of CUR along with bioenhancers. The aims was to utilize the properties of bioenhancers, mucoadhesive properties of CH along with FA mediated active uptake of NPs to improve the oral absorption of CUR. FA functionalized CH-NPs, loaded with CUR and bioenhancers were investigated against hormone refractory prostate cancer with exponential increase in drug uptake in prostate cancer cells and Caco-2 cell monolayers as an evidence of effective oral delivery with enhanced bioavailability.
2. Results
2.1 Conjugated FA–CH characterization by FTIR
FA has been covalently conjugated to CH via its gamma-carboxyl moiety. The FTIR spectra of the FA modified CH showed typical peaks (Fig. 1). The O–H broadened stretching band in the region of 3281 cm−1 was found. IR spectra of the FA conjugated CH, show characteristic absorption bands at 1063 cm−1 which were due to C–O stretching vibrations of the ester formed. The IR spectra of FA conjugated CH shows C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching at nearly 1630 cm−1. Characteristic FT-IR absorption peaks of folic acid at 1450, 1520, and 1605 cm−1 were observed in the spectrum of folic acid–chitosan conjugates.
O stretching at nearly 1630 cm−1. Characteristic FT-IR absorption peaks of folic acid at 1450, 1520, and 1605 cm−1 were observed in the spectrum of folic acid–chitosan conjugates.
2.2 Formulation of nano-bioenhancers loaded with CUR and characterization
A phyto-formulation (CUR–B–CH-NPs) was developed using some important bioenhancers which are also components of “trikatu”, an ayurvedic polyherbal formulation. The traditional formula for “trikatu” as documented in ayurveda is P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N
N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z (1
Z (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In our study we had exploited a modification of “trikatu” in combination with CUR in a ratio CUR
1). In our study we had exploited a modification of “trikatu” in combination with CUR in a ratio CUR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T
T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z (10
Z (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to formulate nano-bioenhancer in pluronic stabilized CH-NPs conjugated with FA. CH is a mucoadhesive carrier suitable for sustained delivery systems, due to limitations of oral delivery of CUR in various attempted systems; we have chosen CH-NPs as it is the most appropriate carrier system which resolves the issue of stability in acidic environment of stomach along with its mucoadhesive properties. CUR loaded nano-cur–bioenhancers were prepared by ionic gelation method using FA grafted CH. Pluronic F68 served as surfactant and stabilizer for the CH-NPs. The CH-NPs are characterized by the size and zeta potential represented in diagram for comparing the size and zeta potential of CH-NPs, CUR–CH-NPs and CUR–B–CH-NPs by dynamic light scattering using Zetasizer. The size of NPs varies from 250 nm to 320 nm and zeta potential was 30 to 36 mV. The data has been represented in Table 1.
1) to formulate nano-bioenhancer in pluronic stabilized CH-NPs conjugated with FA. CH is a mucoadhesive carrier suitable for sustained delivery systems, due to limitations of oral delivery of CUR in various attempted systems; we have chosen CH-NPs as it is the most appropriate carrier system which resolves the issue of stability in acidic environment of stomach along with its mucoadhesive properties. CUR loaded nano-cur–bioenhancers were prepared by ionic gelation method using FA grafted CH. Pluronic F68 served as surfactant and stabilizer for the CH-NPs. The CH-NPs are characterized by the size and zeta potential represented in diagram for comparing the size and zeta potential of CH-NPs, CUR–CH-NPs and CUR–B–CH-NPs by dynamic light scattering using Zetasizer. The size of NPs varies from 250 nm to 320 nm and zeta potential was 30 to 36 mV. The data has been represented in Table 1.
| Formulation | Particle size (nm) | PDI | Zeta potential (mV) | % EE |
|---|---|---|---|---|
| CH-NP | 189.2 ± 1.5 | 0.21 | 48.1 ± 2.7 | — |
| CUR–CH-NPs | 271.8 ± 2.5 | 0.39 | 32.4 ± 1.5 | 52 ± 2.5 |
| CUR–B–CH-NPs | 338.2 ± 3.7 | 0.35 | 36.0 ± 3.1 | 46 ± 1.3 |
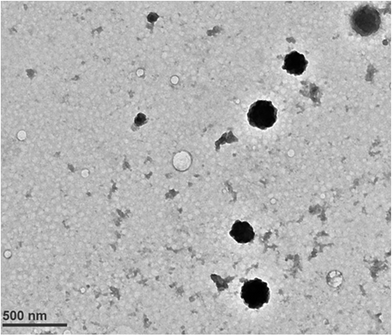
The size and morphology of CUR–B–CH-NPs was further confirmed by TEM and the images supported the zetasizer data that the CUR–B–CH-NPs had a size of <300 nm. Furthermore, TEM images of CUR–B–CH-NPs showed that NPs formed are spherical and uniform. In CUR–B–CH-NPs, the matrix was darker and a contrast was observed inside the spherical matrix of the NPs (Fig. 2).
2.3 Drug entrapment and in vitro release studies
The formulation optimization was carried out for estimation of amount of CUR can be encapsulated in void CH-NPs without any precipitation. The CH-NPs can retain 0.4% w/v of CUR and no precipitation was detected at this concentration. With increase in the percentage of CUR up to 0.6%, marked precipitation was observed which become more evident at higher concentrations. The maximum entrapment efficiency (% EE) of CUR in CUR–CH-NPs and CUR–B–CH-NPs was found 42%.In vitro release profile of CUR from CUR–CH-NPs and CUR–B–CH-NPs is well summarized in Fig. 3. The release pattern of CUR in CUR–CH-NPs and CUR–B–CH-NPs was compared through graphical representation. The percent cumulative drug released in 24 h by CUR–CH-NPs was 64% and by CUR–B–CH-NPs was 53%. As the natural products degraded in acidic medium, therefore for oral delivery we also ensure the extent of drug released in acidic medium which was found to be 18% in CUR–CH-NPs and 14% in CUR–B–CH-NPs. This release kinetic data served as a strong evidence for CH-NPs are well suitable for oral delivery carriers and are capable of rescuing CUR from acid degradation, facilitating the CUR release in the intestine.
 | ||
| Fig. 3 Release of curcumin from plain CUR, CUR–CH-NPs in comparison with CUR–B–CH-NPs. Data represented as mean ± S.D. (n = 3). | ||
2.4 In vitro studies
2.5 Cell uptake study of nano-cur–bioenhancers
2.6 Cell migration assay
Cell migration and cell interaction can be studied as an important feature for distinguishing invasiveness of malignancies. PC3 cells are highly aggressive with high proliferation. Therefore, anti-metastatic potential was assessed by cell migration assay to draw a comparative profile of CUR, CUR–CH-NPs and CUR–B–CH-NPs. This can be related to inhibition of cancer metastasis and neoangiogenesis in invasive prostate cancer. The effect on cell migration was assessed by scratch assay, PC-3 cells treated with sub IC50 concentrations of CUR, CUR–CH-NPs and CUR–B–CH-NPs were observed after 48 h to evaluate the extant of cell proliferation. The gap made by scratch manifested as 100% in images at the start of experiment at 0 h. After completion of 48 h, the gap between the scratch margins was much retained in CUR–B–CH-NPs and CUR–CH-NPs as compared to CUR and control (Fig. 7). | ||
| Fig. 7 Represents the effect of CUR–CH-NPs and CUR–B–CH-NPs on the migration of PC3 cells after 48 h of treatment. Data represented as mean ± S.D. (n = 3). | ||
2.7 Pharmacokinetic study
CZP loaded in FA conjugated CH-NPs stabilized by pluronic F68 was designed for the purpose of improving the pharmacokinetics profile of the CUR and to accommodate the CZP combination in polymeric NPs with improved performance of oral delivery of CUR. The CZP and CUR–B–CH-NPs with oral dose 100 mg kg−1 was administered to male Wistar rats. The mean resistance time MRT for CUR solution, CZP and CUR–B–CH-NPs was found to be 7.6 ± 4.6, 9.5 ± 0.40 and 11.23 ± 2.3 respectively. There was a significant increase in Cmax in CUR–B–CH-NPs about 7.7 times (6080.85 ± 11.1) as compared to CUR solution (789 ± 5.4) and 1.9 times of CZP (1520 ± 15.75). The bioavailability of CUR was assessed by estimation of AUC; the AUC had increased too many folds nearly 6 times in CUR–B–CH-NPs (45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200.97 ± 24.3) than CUR (7456.24 ± 45.67) and 4 times enhanced than CZP (10
200.97 ± 24.3) than CUR (7456.24 ± 45.67) and 4 times enhanced than CZP (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800.6 ± 10.5). The clearance of CUR was decreased in CUR–B–CH-NPs (287.6 ± 14.3) whereas CUR solution and CZP was found to be 802.3 ± 71.2 and 567.4 ± 34.2 respectively.
800.6 ± 10.5). The clearance of CUR was decreased in CUR–B–CH-NPs (287.6 ± 14.3) whereas CUR solution and CZP was found to be 802.3 ± 71.2 and 567.4 ± 34.2 respectively.
3. Materials and methods
3.1 Materials
Curcumin (CUR) was procured from Merck. Chitosan (CH) (70% deacetylated), folic acid (FA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), Tween 80 and Span 85 were purchased from Sigma Aldrich (St. Louis, USA). Sodium tripolyphosphate (TPP), HPLC grade acetonitrile and acetone were purchased from Spectrochem India. Fetal bovine serum (FBS) was purchased from GIBICO. All other chemicals used were of analytical grade. Milli Q water was used throughout the study was prepared by Milli-Q plus 185 purification system (Bedford, Massachusetts). Piper longum, Piper nigrum and, Zingiber officinale were procured as a fresh material from the local suppliers and were identified by Dr A. K. S Rawat, Head, Pharmacognosy and Ethnopharmacology Division, CSIR-NBRI, India.3.2 Preparation of CZP phytoformulation
The extracts of Piper longum Linn. (P), Piper nigrum L. (N) and Zingiber officinale R. (Z) were prepared in methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (70
water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). These extracts were combined with CUR in methanol to constitute a phytoformula CZP, having CUR
30). These extracts were combined with CUR in methanol to constitute a phytoformula CZP, having CUR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Piper longum Linn.
Piper longum Linn.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Piper nigrum L.
Piper nigrum L.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zingiber officinale R. in a ratio (10
Zingiber officinale R. in a ratio (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), followed by rota-evaporation and lyophilization to obtain powdered combination CZP.
1), followed by rota-evaporation and lyophilization to obtain powdered combination CZP.
3.3 Preparation of FA conjugated CH
FA conjugated CH was prepared by using carbodiimide chemistry. In brief a solution of folic acid and EDC was prepared in 5 ml DMA (molar ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and stirred at room temperature until they were well-dissolved. This mixture was then added to CH solution in (0.5% acetic acid) and stirred at room temperature overnight to conjugate folic acid onto CH molecules. To obtain the conjugate solution was brought to pH 9.0 using NaOH aqueous solution (1.0 M) and centrifuged at 4500 rpm to settle down the folic acid conjugated CH. The precipitate was then dialyzed extensively first against phosphate buffered saline (PBS, pH 7.4) for 3 days. Finally the dried conjugate was obtained by lyophilization.14,28
1) and stirred at room temperature until they were well-dissolved. This mixture was then added to CH solution in (0.5% acetic acid) and stirred at room temperature overnight to conjugate folic acid onto CH molecules. To obtain the conjugate solution was brought to pH 9.0 using NaOH aqueous solution (1.0 M) and centrifuged at 4500 rpm to settle down the folic acid conjugated CH. The precipitate was then dialyzed extensively first against phosphate buffered saline (PBS, pH 7.4) for 3 days. Finally the dried conjugate was obtained by lyophilization.14,28
3.4 Preparation of nano-cur–bioenhancer (CUR loaded FA conjugated CH-NPs)
CUR loaded FA functionalized CH-NPs were prepared by ionic gelation method by charge interaction of the positively charged FA–CH and negatively charged TPP.19 In short, CUR was dissolved in FA–CH solution in 1% acetic acid in a ratio of ((CUR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FA–CH): (1
FA–CH): (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5)). The solution was stabilized by pluronic F68. The ionic gelation was achieved by dropwise addition of 2% (w/v) TPP solution prepared in triple distilled water, assisted by continuous stirring for 5 h at room temperature. The resulting NPs were centrifuged at 10
5)). The solution was stabilized by pluronic F68. The ionic gelation was achieved by dropwise addition of 2% (w/v) TPP solution prepared in triple distilled water, assisted by continuous stirring for 5 h at room temperature. The resulting NPs were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min and finally washed thrice with deionized water and lyophilized with 10% sucrose as a cryoprotectant. Nano-cur–bioenhancer i.e. curcumin with bioenhancers (CUR–B–CH-NPs) co loaded FA–CH nanoparticles were also prepared following same protocol using ionic gelation method.
000 rpm for 20 min and finally washed thrice with deionized water and lyophilized with 10% sucrose as a cryoprotectant. Nano-cur–bioenhancer i.e. curcumin with bioenhancers (CUR–B–CH-NPs) co loaded FA–CH nanoparticles were also prepared following same protocol using ionic gelation method.
3.5 Particle size and zeta potential
The mean particle size, size distribution and zeta potential of CUR–CH-NPs and CUR–B–CH-NPs were determined by a Malvern Zetasizer Nano ZS (Malvern 3000HS, France). Each sample was measured in triplicate.3.6 Transmission electron microscope (TEM)
The particles morphology was examined by TEM. Sample preparation was done by depositing a drop of diluted formulations CUR–CH-NPs and CUR–B–CH-NPs on the copper grid, dried and coated with 1% (w/v) phosphotungstic acid. These films were analysed under a transmission electron microscope and images were taken.3.7 % entrapment efficiency (% EE)
% EE is a function of total amount of CUR loaded in the CUR–CH-NPs and CUR–B–CH-NPs. % EE was determined in the formulations by centrifugation of CUR–CH-NPs and CUR–B–CH-NPs at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 40 min. The supernatant was collected as free drug and pellet as entrapped CUR. The pellet is reconstituted in 200 μl methanol and analyzed by HPLC for CUR content.
000 rpm for 40 min. The supernatant was collected as free drug and pellet as entrapped CUR. The pellet is reconstituted in 200 μl methanol and analyzed by HPLC for CUR content.
The total amount of CUR was the drug loading and % EE was determined as follows:
| % EE = [(Wt − Wf)/Wt] × 100 |
3.8 HPLC analysis
Curcumin content was estimated in by RP-HPLC method in CUR–CH-NPs and CUR–B–CH-NPs was estimated through RP-HPLC method already developed and validated. The HPLC system was equipped with two 10 ATVP binary gradient pumps (Shimadzu), Rheodyne (Cotati, CA, USA) model 7125 injector fitted with a 20 μl loop and SPD-M10 AVP UV detector (Shimadzu). HPLC separation was achieved on a RP-C18 column (250 mm, 4.6 mm, 5 μm, Merck) at 425 nm. Data was processed using Empower software. A mixture of acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) triple distilled water
triple distilled water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glacial acetic acid (700
glacial acetic acid (700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 290
290![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) v/v/v was used as mobile phase and flow rate was 1.0 ml min−1. The mobile phase was filtered and degassed prior to use. Calibration curve of CMN was in the range of 1–10 μg ml−1 and retention time for CMN was about 4 minute.
10) v/v/v was used as mobile phase and flow rate was 1.0 ml min−1. The mobile phase was filtered and degassed prior to use. Calibration curve of CMN was in the range of 1–10 μg ml−1 and retention time for CMN was about 4 minute.
3.9 In vitro release
In vitro release profiling of CUR was carried out to assess the effect of components of phytoformula on release of CUR in plain CUR, CUR–B–CH-NPs in comparison to CUR–CH-NPS using dialysis bag technique at 37 °C using simulated gastric fluid (SGF) of pH 1.5 (2 h) and simulated intestinal fluid (SIF) of pH 7.4 as a release media with some modification. The simulated gastric fluid (pH 1.2) composed of sodium chloride (2.0 g), pepsin (3.2 g) and hydrochloric acid (7.0 ml) volume made up to 1000 ml with water. The simulated intestinal fluid (pH 7.4) contained monobasic potassium phosphate (6.8 g), 0.2 N sodium hydroxide (180 ml) and pancreatin (10.0 g) and volume made up to 1000 ml with water. Lyophilized CUR–CH-NPs and CUR–B–CH-NPs (1 mg ml−1) were dispersed in SGF in a dialysis bag for 2 h, then into SIF and incubated at 37 °C under gentle agitation. The sink condition was maintained by withdrawing a known volume of medium and replacing it by equal amount of fresh medium at a fixed time intervals 0, 30 min, 1 h, 2 h, 4 h, 6 h, 12 h, 24 h and 48 h. The withdrawn sample was analysed after extracting it with 10% ethanol, for CUR content by HPLC method as described earlier.The analysis procedure was same as described in the determination of the total drug content. The measurement was done triplicate.
| (CUR)total cumulative release (%) = released CUR × 100/total encapsulated CUR |
3.10 In vitro cytotoxicity study of nano-bioenhancers
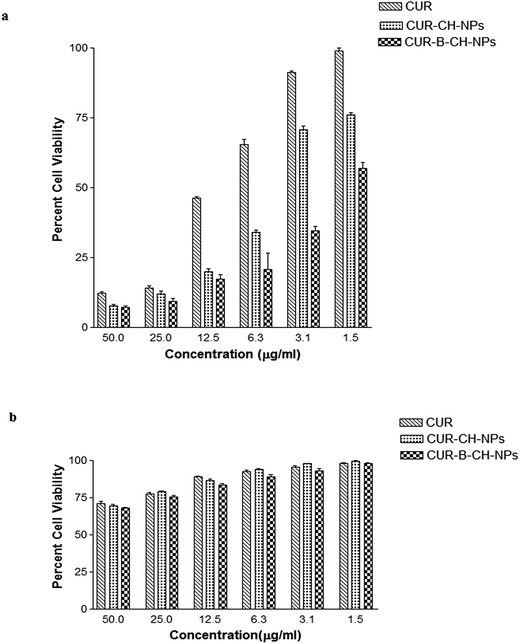
Cytotoxicity of CUR was evaluated on PC3 and HEK cell-lines by MTT assay as described earlier (Anto et al., 2003). The cells were seeded in a 96-well plates with cell density (1 × 103 cells per well) and incubated at 37 °C, 5% CO2 overnight in DMEM media containing 10% FBS. The cells were treated with various concentrations of CUR, CUR–CH-NPs, CUR–B–CH-NPs ranging from 0 to 40 μg ml−1 equivalent CUR and incubated for 48 h. At the end of experiment, MTT dye (8 μl, 5 mg ml−1) was added and plates were incubated for next 4 h, facilitating the formation of MTT-formazan crystal, later dissolved with DMSO. The optical density was measured using Elisa plate reader at 540 nm.3.11 Cell apoptosis study
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per sample were acquired in histogram using a data analysis program CELL Quest. Dead cells and debris were excluded from the analysis by electronic gating of forward and side scatter measurements.
000 cells per sample were acquired in histogram using a data analysis program CELL Quest. Dead cells and debris were excluded from the analysis by electronic gating of forward and side scatter measurements.3.12 Intracellular uptake studies
The cell uptake study by fluorescence microscope was carried out in 6 well culture plates preoccupied by cover slips in each well and incubated at 37 °C until a sub-confluent level attained. Cellular uptake of FITC-labelled CUR–CH-NPs, CUR–B–CH-NPs (100 μg ml−1) was determined after 4 h of incubation with cells using a fluorescence microscope (Olympus-Provis, AX70) attached to an Olympus camera (DP70 Digital). The images were taken at different time intervals 30 minutes, 1 hour and 3 hours to conclude the event of maximum uptake.
The HBSS was replaced by 100 μl CUR–CH-NPs and CUR–B–CH-NPs followed by incubation for 2 h. After 2 h incubation, the formulations were removed from the cells and the cells were washed three with 100 μl PBS. Finally, the cells were lysed by 50 μl 0.5% Trixon-100. The fluorescence intensity was observed at λex 430 nm, λem 485 nm by Microplate Reader (Tecan, Männedorf, Switzerland). The fluorescence intensity of the untreated cells served as negative control. The uptake efficiency was then calculated as:
| Uptake efficiency% = sample O.D − negative control/positive control − negative control |
3.13 Inhibition of cell migration: wound healing assay
Migration and mobility of PC3 cells were assessed using a wound healing assay. Cells were cultured into 12-well plates and grown to upto 90% confluence. This PC3 cell monolayer was then scratched wound using a sterile pipette tip. Scratched cultures were exposed for 4 h to CUR formulations (sub IC50), then cultured in fresh medium for another 48 h. Digitized images of the wound area were captured using a Zeiss Axiovert 40 inverted microscope (Germany).3.14 Pharmacokinetic study
In vivo pharmacokinetic study was conducted on male SD rats overnight fasted and were maintained in standard conditions in respect of animal diet, room temperature (25 °C ± 2 °C) and relative humidity (30% to 70%). The animals were divided into 3 groups (n = 6): plain CUR, CZP and CUR–B–CH-NPs. Each group of rats were administered with dose equivalent to 100 mg kg−1 of CUR orally. Post administration, approximately 1 ml of blood was collected in micro tubes containing heparin at the time intervals 0.25, 0.5, 1, 2, 4, 8, 12, 24 h from the retro-orbital plexus. The blood samples were processed immediately by centrifugation at 2000 rpm, 5 min at 4 °C to harvest plasma and stored at −80 °C, the plasma samples were analysed for CUR content using HPLC method. The plasma samples were prepared by liquid–liquid extraction using TBME.4. Statistical analysis
All results have been expressed as mean ± SD (n = 3). Differences between formulations were compared using one-way analysis of variance (ANOVA) followed by the Turkey–Kramer multiple comparison test, using Graph Pad Instat software (Graph Pad Software Inc., CA, USA). p < 0.05 denotes significance in all cases.5. Discussion
FA conjugation with CH is a complementary attempt to establish a new evolution in oral delivery systems. FA was used as a surface ligand of CH due to stability of folic acid as well as its transcytosis capacity, which impart enhanced cellular uptake of drug.13,28 The conjugation of FA–CH was characterized by FT-IR. The most significant IR frequencies of the FA–CH conjugates are the amide I band (carbonyl stretching) and the amide II band (N–H deformation mode) vibration. The former is typically around 1720–1740 cm−1 and is shifted to lower frequencies by hydrogen bonding (weakening of the force constant due to partial single-bond character). The latter is typically between 1500 and 1550 cm−1 and is believed to shift to the higher frequencies upon hydrogen-bond formation (strengthening of the force constant for deformation vibrations). The nano formulations were carefully optimized for uniformity of size and stability of zeta potential. In addition, zeta potential has been shown to affect the intra-cellular localization of the NPs.31 The particle size of CUR–B–CH-NPs (338.2 ± 3.7) was increased as compared to CUR–CH-NPs (271.8 ± 2.5) with slight increase in zeta potential (36 ± 3.1 mV), whereas the blank CH-NPs were (189.2 ± 1.5) with zeta potential 48.1 ± 2. The positive zeta potential is a net effect of CH encapsulation, the zeta potentials suggests a stable nano-formulation. The size of NPs depends on the concentration of CH and TPP, as the NPs are amalgamated through interaction of NH group of chitosan with the phosphate group of TPP. CH has characteristic feature of stability in acidic medium, which rescues the active ingredients from acidic pH of stomach.15,16 Folic acid is also reported to have stability at gastric and intestinal fluids.13Peeping into the drug transport mechanism via neutral red assay in prostate cancer cells using in vitro cell internalization in PC3 cells shows that CUR influx was increased upto 18.4% in PC3 cells. This is a remarkable feature of bioenhancers like piperine that inhibits the PGP and gingerol influencing the cell membrane permeability and consequently changing the membrane dynamics.9,25,32,33 The drug localization was confirmed by fluorescence microscopy of FITC loaded nano-formulations using DAPI for nucleus staining, the exceptional impact was due to CH as nano-carrier with stabilizer pluronic F68, though the effect was more pronounced in CUR–B–CH-NPs due to CH conjugation with FA. Again, this is clear evidence regarding the modulation of transport mechanism by the polyphenolic components like piperine and gingerol-6 that actively involved in inhibition of P-glycoprotein in CUR–B–CH-NPs, as curcumin when co-administered with piperine in nano-delivery system affect the cellular internalization of CUR. Moreover, it also augments the oral bioavailability of the CUR.25,33 Adding to it, folic acid receptors are expressed on intestinal epithelial cells allowing upregulated transport of folate conjugated nano-delivery systems inside the cells. FA follows two different paths: through facilitated transport via reduced folate carrier distinct routes and by associating with folate receptors at the surface of cell to enter the cells through caveolae-mediated endocytosis.13 The cytotoxicity study on prostate cancer cell-line PC3 showed remarkable depression in the IC50 of the CUR–B–CH-NPs by synergistic effect of polyphenolic bioenhancer in the formulation. PC3 cell line is AR negative and has highly invasive cells.34 We thus expanded our experiments on PC3 cells to elucidate the effect of CUR–B–CH-NPs on hormone independent prostate cancer as compared to CUR–CH-NPs. Though, CUR is active on AR arbitrated cancers but it had faced some limitation regarding cell uptake and bioavailability of the CUR even in formulations mend for oral delivery.23 This was fairly clear in apoptosis results on PC3 cells; CUR–B–CH-NPs raised the percent of apoptotic cells as compared to CUR by 2.3 fold. The mechanistic study of CUR–B–CH-NPs exposed the intrinsic apoptotic pathways involved; these are more related to the polyphenols as complex combination of bioenhancers can trigger different pathways related to apoptosis. Change in mitochondrial membrane potential was evident through JC1 assay, which is significantly involved in apoptosis. CUR–B–CH-NPs reduced the MMP by 2.7 fold indicating MMP disruption as intrinsic apoptosis pathway. The MMP disruption was correlated to the proxidant role of polyphenols that raised the ROS concentration to fatal level in cancer cells. The DCF-DA assay indicated nearly 1.68 fold enhanced ROS level in PC3 cells by CUR–B–CH-NPs in comparison to CUR–CH-NPs at their respective sub IC50 in comparison to positive control H2O2. Previous studies have already generated the blueprints of bioenhancer's effect on single drug in terms of bioavailability. In contradiction, bioenhancers P, N and Z exerted very weak cytotoxic effect on the PC3 cells. It is well supported through preceding experiments that weak phytochemicals in combination attributed to an exceptional synergistic cocktail.35 The oral bioavailability of CUR is a major limitation with the successful delivery of CUR; previous in vitro cell studies draw our confidence in nano-cur–bioenhancers CUR–B–CH-NPs. Also, it can be well predicted from the in vivo pharmacokinetic data that CUR–B–CH-NPs exhibited remarkably improved bioavailability than CUR–bioenhancer combination CZP and CUR solution. On relative comparison of the statistical data detailed a profoundly enhanced AUC by 4 folds and 6 times and Cmax by 1.9 folds and 7.7 folds in CUR–B–CH-NPs than CZP and CUR solution respectively, this may be attributed to the chitosan armouring the CZP to rescue drug from acidic degradation and also facilitates controlled release assisted by surfactant pluronic F68. Pluronic F68 enhances the solubility of the CUR and tangibly contributes by increasing the permeability of the intestinal membrane and cellular internalization of CZP in CH-NPs.15
6. Conclusion
For development of effective therapeutic strategies for patients with hormone-dependent and hormone-refractory disease, CUR with natural bioenhancers was endeavoured to co-deliver in a nanosystem. The results concluded folic acid conjugated CH-NPs are very effective oral delivery carrier for phyto-compounds in respect of particle size, stability and encapsulation efficiency. Adding on this, they support a sustained release profile and efficiently augment the cell uptake by inhibiting drug transporter proteins (P-gps) to achieve improved oral bioavailability of CUR. This strategy of using bioenhancers with drug can be utilized as the effective tool to circumvent the limitation of present chemotherapy especially related to androgen receptor independent prostate cancer. This can be exploited as a very sincere way to reduce the toxicity of drug by cutting down the dose as higher doses of the drug may contribute to toxicity and adverse effects. Newer therapeutic strategies in cancer management continue to emphasize molecular mechanisms.Ethical statement
Animal studies were conducted in accordance with current legislation of institute on animal experiments, and protocol was approved by the ‘Institutional Animal Ethical Committee’ of CSIR-Central Drug Research Institute. They were maintained in climatically controlled rooms and fed with standard rodent food pellet (Lipton India Ltd, Bombay) and water ad libitum. The study was carried out with the guidelines of Council for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India.Conflict of interest
The authors declare they have no competing financial interest.Acknowledgements
The authors are particularly grateful to DST-Inspire, New Delhi, India for the financial support and Director CSIR-NBRI, Lucknow for necessary facilities.References
- J. P. van Brussel, G. J. van Steenbrugge, J. C. Romijn, F. H. Schroder and G. H. Mickisch, Eur. J. Cancer, 1999, 35, 664–671 CrossRef CAS PubMed.
- W. W. Scott, M. Menon and P. C. Walsh, Cancer, 1980, 45, 1929–1936 CAS.
- E. Rivera and H. Gomez, Breast Cancer Res., 2010, 12(suppl. 2), S2 CrossRef PubMed.
- Y. Shi, L. Chen, J. Li, Y. L. Lv, Q. Sun, L. X. Wang and S. C. Jiao, Tumor Biol., 2011, 32, 381–390 CrossRef CAS PubMed.
- C. Duhem, F. Ries and M. Dicato, Oncologist, 1996, 1, 151–158 Search PubMed.
- M. Dean and R. Allikmets, J. Bioenerg. Biomembr., 2001, 33, 475–479 CrossRef CAS PubMed.
- F. Thiebaut, T. Tsuruo, H. Hamada, M. M. Gottesman, I. Pastan and M. C. Willingham, Proc. Natl. Acad. Sci. U. S. A., 1987, 84, 7735–7738 CrossRef CAS.
- N. R. Patel, A. Rathi, D. Mongayt and V. P. Torchilin, Int. J. Pharm., 2011, 416, 296–299 CrossRef CAS PubMed.
- E. Borowski, M. M. Bontemps-Gracz and A. Piwkowska, Acta Biochim. Pol., 2005, 52, 609–627 CAS.
- J. Wu, Y. Lu, A. Lee, X. Pan, X. Yang, X. Zhao and R. J. Lee, J. Pharm. Pharm. Sci., 2007, 10, 350–357 CAS.
- H. L. Wong, R. Bendayan, A. M. Rauth, H. Y. Xue, K. Babakhanian and X. Y. Wu, J. Pharmacol. Exp. Ther., 2006, 317, 1372–1381 CrossRef CAS PubMed.
- K. W. Kang, M. K. Chun, O. Kim, R. K. Subedi, S. G. Ahn, J. H. Yoon and H. K. Choi, Nanomedicine, 2010, 6, 210–213 CrossRef CAS PubMed.
- E. Roger, S. Kalscheuer, A. Kirtane, B. R. Guru, A. E. Grill, J. Whittum-Hudson and J. Panyam, Mol. Pharm., 2012, 9, 2103–2110 CrossRef CAS PubMed.
- M. Sharma, R. Malik, A. Verma, P. Dwivedi, G. S. Banoth, N. Pandey, J. Sarkar, P. R. Mishra and A. K. Dwivedi, J. Biomed. Nanotechnol., 2013, 9, 96–106 CrossRef CAS PubMed.
- H. Hosseinzadeh, F. Atyabi, R. Dinarvand and S. N. Ostad, Int. J. Nanomed., 2012, 7, 1851–1863 CrossRef CAS PubMed.
- N. Khan, D. J. Bharali, V. M. Adhami, I. A. Siddiqui, H. Cui, S. M. Shabana, S. A. Mousa and H. Mukhtar, Carcinogenesis, 2014, 35, 415–423 CrossRef CAS PubMed.
- H. Jing, Z. Guo, W. Guo, W. Yang, P. Xu and X. Zhang, Bioorg. Med. Chem. Lett., 2012, 22, 3418–3424 CrossRef CAS PubMed.
- W. Guo, H. Jing, W. Yang, Z. Guo, S. Feng and X. Zhang, Bioorg. Med. Chem. Lett., 2011, 21, 6446–6450 CrossRef CAS PubMed.
- P. Calvo, C. Remunan-Lopez, J. L. Vila-Jato and M. J. Alonso, Pharm. Res., 1997, 14, 1431–1436 CrossRef CAS.
- Y. Hu, Y. Ding, D. Ding, M. Sun, L. Zhang, X. Jiang and C. Yang, Biomacromolecules, 2007, 8, 1069–1076 CrossRef CAS PubMed.
- P. Shukla, A. K. Verma, J. Dewangan, S. K. Rath and P. R. Mishra, RSC Adv., 2015, 5, 57006–57020 RSC.
- H. Warenius, A. Howarth, L. Seabra, L. Kyritsi, R. Dormer, S. Anandappa and C. Thomas, J. Exp. Ther. Oncol., 2008, 7, 237–254 CAS.
- T. Dorai, Y. C. Cao, B. Dorai, R. Buttyan and A. E. Katz, Prostate, 2001, 47, 293–303 CrossRef CAS PubMed.
- P. Anand, A. B. Kunnumakkara, R. A. Newman and B. B. Aggarwal, Mol. Pharm., 2007, 4, 807–818 CrossRef CAS PubMed.
- R. K. Bhardwaj, H. Glaeser, L. Becquemont, U. Klotz, S. K. Gupta and M. F. Fromm, J. Pharmacol. Exp. Ther., 2002, 302, 645–650 CrossRef CAS PubMed.
- C. K. Atal, R. K. Dubey and J. Singh, J. Pharmacol. Exp. Ther., 1985, 232, 258–262 CAS.
- D. Chanda, K. Shanker, A. Pal, S. Luqman, D. U. Bawankule, D. Mani and M. P. Darokar, J. Toxicol. Sci., 2009, 34, 99–108 CrossRef CAS PubMed.
- S. J. Yang, F. H. Lin, K. C. Tsai, M. F. Wei, H. M. Tsai, J. M. Wong and M. J. Shieh, Bioconjugate Chem., 2010, 21, 679–689 CrossRef CAS PubMed.
- A. Mathew, T. Fukuda, Y. Nagaoka, T. Hasumura, H. Morimoto, Y. Yoshida, T. Maekawa, K. Venugopal and D. S. Kumar, PLoS One, 2012, 7, e32616 CAS.
- C. F. Chignell, P. Bilski, K. J. Reszka, A. G. Motten, R. H. Sik and T. A. Dahl, Photochem. Photobiol., 1994, 59, 295–302 CrossRef CAS PubMed.
- S. Prabha, W. Z. Zhou, J. Panyam and V. Labhasetwar, Int. J. Pharm., 2002, 244, 105–115 CrossRef CAS PubMed.
- C. K. Atal, U. Zutshi and P. G. Rao, J. Ethnopharmacol., 1981, 4, 229–232 CrossRef CAS PubMed.
- T. Nabekura, S. Kamiyama and S. Kitagawa, Biochem. Biophys. Res. Commun., 2005, 327, 866–870 CrossRef CAS PubMed.
- S. Tai, Y. Sun, J. M. Squires, H. Zhang, W. K. Oh, C. Z. Liang and J. Huang, Prostate, 2011, 71, 1668–1679 CrossRef CAS PubMed.
- R. Kumar, V. Verma, A. Jain, R. K. Jain, J. P. Maikhuri and G. Gupta, J. Nutr. Biochem., 2011, 22, 723–731 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra17599b |
| This journal is © The Royal Society of Chemistry 2016 |