Sorption of arsenic onto Ni/Fe layered double hydroxide (LDH)-biochar composites†
Shengsen Wanga,
Bin Gao*a,
Yuncong Lib,
Andrew R. Zimmermanc and
Xinde Caod
aDepartment of Agricultural and Biological Engineering, University of Florida, Gainesville, FL 32611, USA. E-mail: bg55@ufl.edu; Tel: +1-352-392-1864 ext. 285
bTropical Research and Education Center, Soil and Water Science Department, University of Florida, Homestead, FL 33031, USA
cDepartment of Geological Sciences, University of Florida, Gainesville, FL 32611, USA
dSchool of Environmental Science and Engineering, Shanghai Jiaotong University, Shanghai 200240, China
First published on 5th February 2016
Abstract
Biochar is a carbon-enriched material that has been investigated for use as a remediation agent for environmental contaminants. However, in order for biochar to see practical use for metal removal, it has to be modified to improve its sorption efficiency. Layered double hydroxides (LDHs) are robust sorbents for removal of a wide array of contaminants. Thus, two LDH-biochar composites were produced by (1) pyrolysis of Ni/Fe-LDH-modified pine feedstock (NFMF), and (2) precipitation of LDHs onto pristine biochars (NFMB). Both composites were characterized and tested for their ability to remove arsenate [As(V)] from aqueous solution. X-ray diffraction, X-ray photoelectron spectroscopy, scanning electron microscopy, and energy-dispersive X-ray analyses suggested that Ni/Fe-LDH had a layered structure that was anchored on the carbonaceous surface in both NFMF and NFMB. The maximum As(V) sorption capacity of NFMF and NFMB (1.56 g kg−1 and 4.38 g kg−1, respectively) was greatly enhanced over that of the unmodified one. The results, such as increased sorption at lower solution pH, indicated that electrostatic attraction and surface complexation with hydroxyl (–OH) groups were the main As(V) sorption mechanisms for both sorbents. The good stability and As(V) sorption make Ni/Fe-LDH modified biochars high-potential sorbents for environmental remediation.
1. Introduction
Removal of arsenate (As(V)) from aqueous solutions by sorption technology is a prevailing remediation method because the adsorption approach usually has high removal efficiency and the advantage of convenient handling.1,2 Extensive efforts have been made to develop optimized sorbents for As(V) removal. Double metal-based layered double hydroxides (LDHs) have attracted increasing interest for their high removal capacity for As(V) and other anionic contaminants.3 LDHs are formed by partial structural substitution of divalent cations by trivalent cations in the octahedral layer of divalent metal hydroxides.4 Various anions, such as nitrate, chlorine, carbonate, can be intercalated between the two layers which imbue the LDHs differing sorption capacities.5,6 Large surface area, good anion exchange capacity, good thermal stability, reversibility of structure, and flexibility of metal composition also make LDHs attractive sorbents for environmental remediation.3,6,7The sorption of As(V) by LDHs has been attributed to several mechanisms including structure reconstruction,5,8 anion exchange, and surface complexation.3,6,9 For example, Huang et al.9 attributed As(V) sorption by Mg/Al-LDH intercalated with carbonate and chloride to ion exchange where interlayer anions was exchanged by As(V). Goh et al.6 found nitrate (NO3−) intercalation of Mg/Al-LDH to increase As(V) sorption through the formation of outer layer and inner layer complexes via ion exchange and surface complexation, respectively.10
Colloidal or nanosized LDHs are usually synthesized using wet chemistry methods by co-precipitation of mixed metals in alkaline conditions followed by proper thermal treatment. For example, divalent magnesium (Mg) and trivalent aluminum (Al) are common cations to make LDH. The properties of resulting Mg/Al-LDH are largely dependent on molar ratio of cations, medium pH, calcination temperature, and intercalation anions.3,9 Other divalent cations (Ni2+, Cu2+, Co2+) and trivalent cations (Fe3+) have replaced Mg and Al respectively to obtain new LDH species for arsenic removal, e.g., Mg/Fe-LDH (precipitated with Na2CO3/NaOH and aged at 110 °C)11 and Cu/Mg/Fe-LDH.12 Ni/Fe-LDH has also been synthesized for As(V) removal which has greater As(V) removal capacity than Mg/Al-LDH.13
Environmental application of colloidal or nanosized adsorbents such as LDHs are generally most effective when they are joined to larger particles, preferable ones that are inexpensive, environmentally recalcitrant, and have additional potential environmental benefits. Biochar is such a material. It is a pyrogenic carbonaceous material that has been commonly used for carbon sequestration and soil amelioration, and recently has been used as a support framework for nanoscale particles to reduce aggregation and increase surface area.1,14–17 Thus, this study explored the potential for biochar to serve as a support for LDLs in the remediation of As(V). Previously, Mg/Al-LDH-biochar composites were synthesized with enhanced performance for phosphate removal.18,19 Because arsenic resembles phosphorus chemically, we propose LDH may be able to remove As(V) as well.
The overall objective of this research was to prepare Ni/Fe-LDH-biochar composites with two synthesis methods and evaluate their performance in As(V) sorption. The additional goal was, through characterization of the materials and modeling of the sorption thermodynamics and kinetic data, to identify possible mechanisms for As(V) sorption.
2. Materials and methods
2.1 Reagents
Reagent grade of sodium arsenate dibasic heptahydrate (Na2HAsO4·7H2O), ferric chloride hexahydrate (FeCl3·6H2O), nickel nitrate (Ni(NO3)2), granular sodium hydroxide (NaOH), and sodium carbonate (Na2CO3) from Fisher Scientific were used in this study. All chemicals were dissolved with 18.2 MΩ cm nanopure water (Nanopure®, Barnstead/Thermolyne Corp, Dubuque, IA).2.2 Biochar preparation
LDH-biochar composites were produced by modifying the biochar with LDH synthesis method previously described.13 The feedstock, loblolly pine (Pinus taeda) wood, was oven dried overnight at 80 °C, then milled into <2 mm fragments with a mechanic miller and particles of size between 0.425 and 2 mm were collected. To synthesize the pre-pyrolysis Ni/Fe-LDH-modified feedstock (NFMF), 25 g feedstock was added to 100 mL of solution containing 5.8 g Ni(NO3)2 (0.02 mol) and 5.4 g FeCl3·6H2O (0.02 mol). The feedstock was allowed to soak in solution for two hours with vigorous agitation. Then, 3.2 g (0.08 mol) NaOH and 4.24 g (0.04 mol) Na2CO3 were then added, leading to the formation of a precipitate, which was rinsed with DI water and dried at 80 °C overnight. The dried feedstock loaded with precipitate was loaded into a GSL 1100X tube furnace (MTI Corporation, Richmond, CA) and pyrolyzed in constantly purged N2 environment at a peak temperature of 600 °C for one hour.To make post-pyrolysis Ni/Fe-LDH-modified biochar (NFMB), 5 g of the pristine biochars (PB) produced at peak temperature of 600 °C from pine wood biomass prepared as described above were added to 25 mL DI water and agitated with a magnetic stirrer and continuously purged with N2 gas for 30 min. Next, 0.02 mol Ni(NO3)2 and 0.04 mol FeCl3·6H2O were dissolved in biochar suspension, which was then added to 250 mL 1.5 M NaOH preheated to 80 °C continuously purged with N2 gas. After 30 min stirring, the resulting biochar composites were vacuum filtered (<0.22 μm) and dried at 80 °C for 12 hour.
2.3 LDH-biochar composites surface characterization
Nitrogen gas sorption and Brunauer–Emmett–Teller (BET) theory was used to obtain specific surface areas with a NOVA 1200 analyzer (QUANTACHROME).20,21 Elemental composition of biochar surfaces and valence states were analyzed by X-ray photoelectron spectroscopy (XPS) with a PHI 5100 series ESCA spectrometer (Perkin-Elmer). Minerals and LDH present on biochar surfaces were characterized using a Philips APD 3720 X-ray diffractometer (XRD) (Philips Electronic Instruments, Mahwah, NJ) equipped with a CuKα radiation source with diffraction angles between 2 and 80°. Surface morphology was examined by scanning electron microscopy (SEM) using a JEOL JSM-6400 Scanning Microscope (JEOL, Japan). Energy dispersive X-ray spectroscopy (EDS, Oxford Instruments Link ISIS) was used to determine surface elemental composition, and distribution of Fe and Ni on biochars surfaces.2.4 LDH-biochar composite adsorption kinetics and isotherms
To examine As adsorption kinetics, 0.05 g of biochar was added to 20 mL As(V) (20 mg L−1) solution in 68 mL digestion vessels (Environmental Express) at room temperature (22 ± 0.5 °C). Thus, adsorbent concentration is equivalent to 2.5 g L−1 for all treatments. The pH for the kinetics and isotherm studies was adjusted to 7.5. The vessels with sorption mixtures, all at 2.5 g L−1, were placed onto a rotary shaker and agitated at 40 rpm until sampled at intervals of 0, 0.5, 1, 2, 4, 8, 12, 24 and 48 h. Samples were immediately filtered through 0.22 μm pore size nylon membrane filters (GE cellulose nylon membrane) and filtrate was collected for As(V) analysis via inductively coupled plasma-atomic emission spectrometry (ICP-AES, Perkin-Elmer Plasma 3200). The As(V) sorption was calculated as the difference between As(V) concentration in initial and final solutions.Sorbent isotherms were constructed by varying ratios of As(V) and sorbents using 20 mL As(V) solution (0–40 mg L−1) containing 0.05 g biochar in 68 mL digestion vessels (Environmental Express). After 24 h agitation, determined to be sufficient time to reach apparent equilibrium, the sorption was terminated and final suspensions were passed through 0.22 μm pore size nylon membrane filters. Again, sorbed As(V) was calculated as the difference between As concentrations in initial and final solutions. The As loaded sorbents (from the 40 mg L−1 solutions) were collected and dried in as oven at 80 °C for surface characterization.
To determine desorption capacity, As(V) was loaded onto sorbents by placing 0.1 g of sorbents in 40 mL of 50 mg L−1 As(V) solution. After sorption (24 h), suspensions were centrifuged for 10 min and the supernatants were collected. Then, 40 mL of 0.1 M NaOH solution were added to the As(V)-loaded sorbents agitated for 48 h. Subsamples were collected at different intervals to monitor desorption progress over time. After this period, sorbents were rinsed with DI water several times, and used for As(V) regeneration experiments by adding 40 mL of 50 mg L−1 As(V) at pH 7.5. The regeneration process was repeated twice.
To examine the effects of pH on sorbent characteristics, an As(V) solution (50 mg L−1) was adjusted to pH 3–9 with 0.1 M NaOH or HCl. The sorption procedure followed the batch experiment described previously and was terminated after 24 h.
3. Results and discussion
3.1 Physical properties of LDH-biochar composites
The bulk Ni and Fe content of the sorbent prepared by pre-pyrolysis Fe–Ni modified feedstock (NFMF) was 5.85 and 5.48 weight% and that of the post-pyrolysis Ni–Fe modified biochars (NFMB) was 4.04 and 1.74 weight%, equivalent to Ni/Fe molar ratio of 1.0 and 2.2 for NFMF and NFMB, respectively (Table 1). C content dropped from 85.8% in PB to 55.3 and 78.4% in NFMF and NFMB, respectively. BET surface area decreased by 47.9% in NFMF after loading of Ni–Fe minerals onto PB prior pyrolysis, whereas surface area was increased by 15.5% in NFMB.| C | N | Ni | Fe | BET surface area m2 g−1 | |
|---|---|---|---|---|---|
| Mass% | |||||
| PB | 85.8 | 0.4 | <0.01 | 0.02 | 334.8 |
| NFMF | 55.3 | 0.6 | 5.5 | 5.9 | 174.4 |
| NFMB | 78.4 | 0.6 | 1.7 | 4.0 | 387.0 |
The sorbents had XRD peaks with d spacing at 7.495, 3.751, 2.590, 1.934, 1.506 Å and 7.867, 3.984, 2.633, 1.533 Å for NFMF and NFMB, respectively (Fig. 1a and c). These patterns matched the characteristic XRD peaks of Ni/Fe-LDH reported previously.22 The regularity of d spacing indicates the crystals have layer structure.5 However, the d spacing for two sorbents were not identical, probably due to expansion or shrinking of the interlayer spacing resulting from anions of different sizes between layers, e.g., NO3−, Cl−, and CO32− in NFMF, and NO3− and Cl− in NFMB. The Ni/Fe-LDH in NFMF was more crystalline than that in NFMB, as indicated by sharper and narrower diffraction peaks in the former. Binding energies of Fe2p 1/2 and 3/2, determined by XPS, were 725.90–725.99 and 711.26–712.00 eV, respectively, in both NFMF and NFMB (Fig. S1, ESI†), corresponding to involvement of trivalent Fe.14 XPS spectra also revealed that binding energies of Ni 2p 3/2 and 1/2 were 856.62–856.89 and 874.35–874.76 eV respectively, in both sorbents, corresponding to divalent Ni.23 Thus, there was no difference between sorbents with respect to the valence states of Ni and Fe. Besides LDH, other crystallites were identified in NFMF at d spacing of 2.063, 1.786, and 1.265 Å, assigned to awaruite (Ni2Fe or Ni3Fe), and d spacing at 2.409 Å, assigned to NiO. The formation of awaruite and NiO is likely due to the presence of excess Ni.
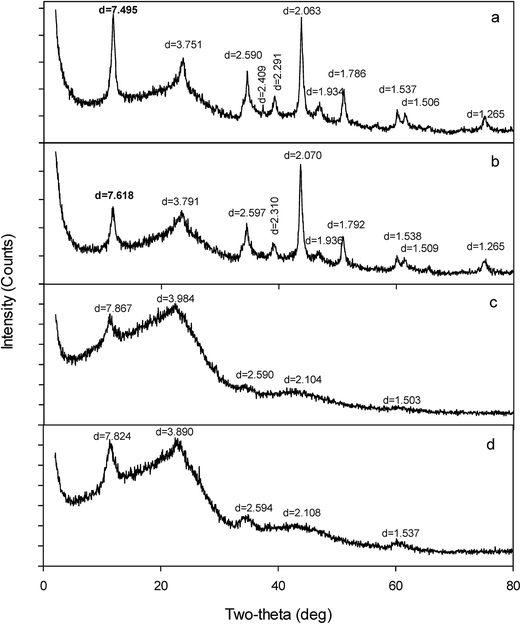 | ||
| Fig. 1 XRD diffraction patterns of NFMF (a), As-loaded NFMF (b), NFMB (c) and As(V)-loaded NFMB (d). | ||
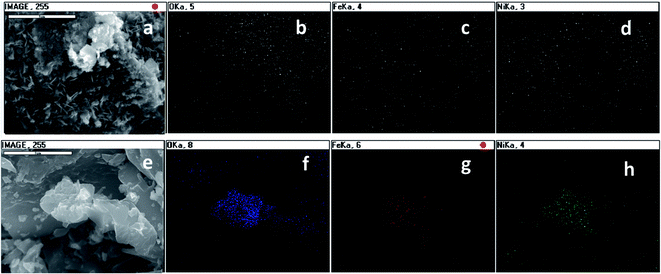
Surface morphology visualized with SEM, revealed that the LDH was associated with the biochar surface matrix (Fig. 2). The LDH on the NFMF suggested a three dimensional network on the carbonaceous surfaces whereas the LDH of NFMB was relatively poorly structured (Fig. 2e and f). EDS mapping indicated that the Fe and Ni in NFMF was similarly distributed while O was concentrated in separate regions on NFMF surfaces. This implies formation of two distinct Ni/Fe crystal types in NFMF, in agreement with the XRD results (Fig. 3). In NFMB, Fe and Ni distributions were almost identical (Fig. 3), supporting the conclusion that LDH was the predominant Ni/Fe phase formed in NFMB.
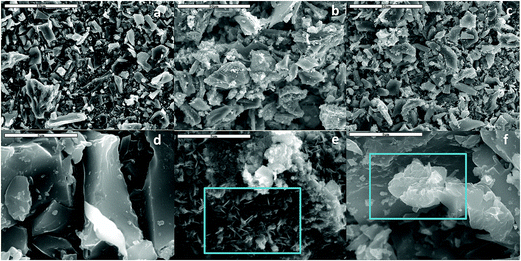 | ||
Fig. 2 SEM for pristine pinewood derived biochars (PB) (a and d), NFMF (b and e), and NFMB (c and f) at ×1000 (a–c) and ×10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (d–f). 000 (d–f). | ||
3.2 As(V) sorption by LDH-biochar composites
The sorption of As(V) by both sorbents increased with contact time and reached apparent equilibrium after 12 h (Fig. 4a). This relatively slow As(V) sorption rate suggests a specific sorption process such as chemisorption. The three mathematical models used to simulate the kinetics data all reproduced the data well, each with R2 above 0.94 for both sorbents (Table 2). However, the As(V) sorption kinetics data for NFMB was best fit by second order kinetics model (R2 = 0.991), whereas the NFMF sorption data was best fit by the Elovich model (R2 = 0.992).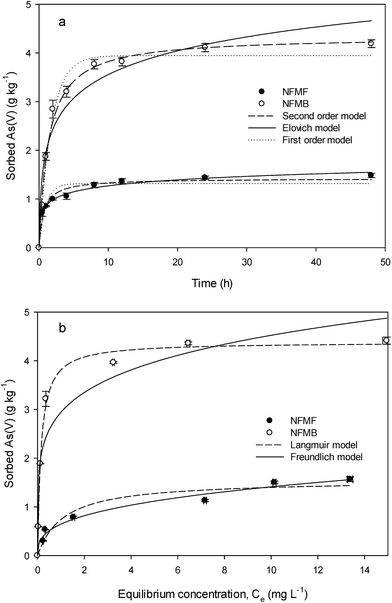 | ||
| Fig. 4 Kinetics (a) and isotherms (b) for aqueous sorption of As(V) onto pre- and post-pyrolysis loaded Ni/Fe-LDH-biochar composites (NFMF and NFMB, respectively). | ||
| Model/equations | Sorbents | Parameter 1 | Parameter 2 | R2 |
|---|---|---|---|---|
| a Note: As(V) sorption kinetics and isotherms parameters from our previous work.15 The data was shown here for comparison purposes. | ||||
| Kinetics | ||||
| First-order, qt = qe(1 − e−k1t) | PBa | qe = 0.129 g kg−1 | k1 = 1.66 h−1 | 0.814 |
| NFMF | qe = 1.32 g kg−1 | k1 = 1.06 h−1 | 0.906 | |
| NFMB | qe = 3.95 g kg−1 | k1 = 0.571 h−1 | 0.984 | |
| Second-order, qt = k2qe2t/(1 + kqet) | PBa | qe = 0.139 g kg−1 | k2 = 16.2 kg g−1 h−1 | 0.900 |
| NFMF | qe = 1.42 g kg−1 | k2 = 1.05 kg g−1 h−1 | 0.968 | |
| NFMB | qe = 4.34 g kg−1 | k2 = 0.171 kg g−1 h−1 | 0.991 | |
Elovich, qt = β−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(βαt + 1) ln(βαt + 1) |
PBa | α = 7.86 g kg−1 h−1 | β = 66.5 kg g−1 | 0.961 |
| NFMF | α = 22.8 g kg−1 | β = 5.63 g kg−1 | 0.992 | |
| NFMB | α = 10.1 g kg−1 | β = 1.43 g kg−1 | 0.942 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Isotherm | ||||
| Langmuir, S = SmaxKC/(1 + KC) | PBa | Smax = 0.201 g kg−1 | K = 0.316 L g−1 | 0.991 |
| NFMF | Smax = 1.56 g kg−1 | K = 0.910 L g−1 | 0.943 | |
| NFMB | Smax = 4.38 g kg−1 | K = 6.71 L g−1 | 0.995 | |
| Freundlich, S = KfCn | PBa | n = 0.332 | Kf = 0.0681 g(1−n) Ln kg−1 | 0.995 |
| NFMF | n = 0.340 | Kf = 0.646 g(1−n) Ln kg−1 | 0.980 | |
| NFMB | n = 0.189 | Kf = 2.91 g(1−n) Ln kg−1 | 0.907 | |
Batch sorption isotherms showed L-curve for NFMF and NFMB, indicating that both sorbents have high affinity for As(V) (Fig. 4b). The equilibrium adsorption capacities of both sorbent increased with increasing As(V) concentration in the range of 0 to 2 mg L−1 and reached maximum sorption capacities above that range. The NFMF and NFMB isotherm data were best fit with the Freundlich model and Langmuir model, respectively (R2 as 0.980 and 0.995, respectively). The Langmuir model showed the maximal As(V) sorption capacity of NFMB (4.38 g kg−1) to be about three times that of NFMF (1.56 g kg−1).
The Langmuir adsorption model assumes monolayer coverage by the sorbate over a homogeneous sorbent surface composed of a finite number of adsorption sites with equal adsorption activation energies24,25 whereas the Freundlich model assumes sorption onto a heterogeneous surfaces. Thus, NFMF has more than one As(V) sorption mechanisms, since it was best fitted with Freundlich model, which may ascribe to different minerals formed on the NFMF. In Freundlich models, the lower n value for NFMB suggested a higher affinity between sorbent and sorbate.
3.3 LDH-biochar composite As(V) desorption
Desorption ability of As(V) loaded onto sorbents is an important when considering the suitability of a sorbent for environmental reclamation. Sodium bicarbonate (NaHCO3) and sodium hydroxide (NaOH) are two commonly used agents to remove As(V) from sorbents, with desorption efficiency between 85 and 99% considered a requirement for practical use.26,27 In this study, 0.1 M NaOH was found to desorb 84% and 65% of initially sorbed As(V) onto NFMB and NFMF, respectively. As(V) desorption curves showed two phases, with a very rapid phase within the first 4 h, followed by a slower phase between 4 and 24 h, after which there was no further desorption (Fig. 5). Following this, the sorbents were used for another two cycles of As(V) sorption/desorption, which resulted in 91% and 92% of original sorption capacity for NFMB, and 95% and 94% for NFMF, respectively. It is noted that the residual fraction which was not displaced by NaOH was most likely retained by specific chemo-sorption. For example, the residual fraction of As(V) which could not be desorbed from Mg/Al-LDH, was ascribed to inner-sphere complexation of As(V).6 These results indicate As(V) can be easily desorbed and has good regeneration potential for future use.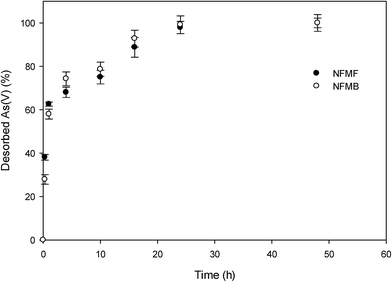 | ||
| Fig. 5 As(v) desorption in 0.1 M NaOH solution as a function of time from As(v) laden NFMF and NFMB. | ||
3.4 Mechanisms of As(V) sorption by LDH-biochar composites
Because of very limited sorption capacity for As(V), e.g., 0.200–0.265 g kg−1 by pristine pine derived biochars,1,15 the improved sorption capacity for both NFMF (1.56 g kg−1) and NFMB (4.28 g kg−1) can be attributed to Ni/Fe-LDH associated with the biochar. Further, XPS spectra of As(V) loaded sorbents confirms uptake of As(V) by NFMF and NFMB (Fig. S2 and S4, ESI†).Results of the kinetics, isotherm and desorption studies suggest that multiple mechanisms (anion exchange and surface complexation) were involved in As(V) sorption. After As(V) sorption, the XRD d spacing of NFMB, 7.867 and 3.984 Å, decreased to 7.824 and 3.890 Å, and that of NFMF, at 7.495 Å, increased to 7.618 Å, respectively. This indicates As(V) may exchange with anions (Cl−, NO3− and/or CO32−) in the interlayers of Ni/Fe-LDH in both sorbents (Fig. S3, ESI†). In addition, the lower As(V) sorption capacity of NFMF may be related to its exchange of intercalated Cl− and CO32− in the interlayers of Ni/Fe-LDH by HAsO42− NFMF. Because CO32− is known to compete As(V) for sorbent,26 the released CO32− decreased As(V) sorption. This phenomenon was proposed to occur in previous work with CO32− intercalated Mg/Al-LDH,13 and implies the possible occurrence of anion exchange in the interlayer.
Surface complexation of As(V) with hydroxyl (–OH) functional groups on biochar surfaces and protonated hydroxyl groups bound to LDH metals is another likely As(V)-LDH-biochar complex sorption mechanism. Although complexation may occur between As(V) and –OH on carbonaceous surfaces, the absolute sorption is quite low and may be negligible when compared to LDH. The LDH surfaces, with a point of zero charge (pHpzc) above 10,28 are net positively charged due to protonation at the experimental pH (7.5). The increased As(V) sorption at lower pH may be related to higher degree of protonation of –OH (Fig. 6). It is presumed, then, that As(V) form specific inner-sphere complexes with LDH hydroxyl functional groups. Potential mechanisms for metal-bonded hydroxyl groups to form complexation with HAsO42− include:6,29
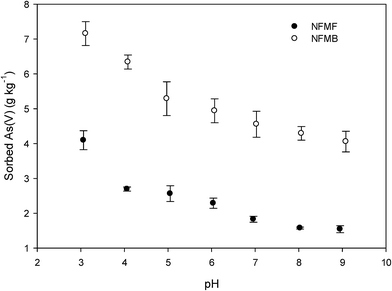 | ||
| Fig. 6 Sorption of As(V) at equilibrium concentration of 50 mg L−1 onto NFMF and NFMB at different pHs. | ||
Monodentate mononuclear:
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ni/Fe–OH2+ + HAsO42− → Ni/Fe–OH2+ + HAsO42− → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ni/Fe–HAsO4− + H2O Ni/Fe–HAsO4− + H2O
| (1) |
Bidentate mononuclear:
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ni/Fe–(OH2+)2 + HAsO42− → Ni/Fe–(OH2+)2 + HAsO42− → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ni/Fe–HAsO4 + 2H2O Ni/Fe–HAsO4 + 2H2O
| (2) |
Bidentate binuclear:
2(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ni/Fe–OH2+) + HAsO42− → ( Ni/Fe–OH2+) + HAsO42− → (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ni/Fe)2 Ni/Fe)2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) HAsO4 + 2H2O HAsO4 + 2H2O
| (3) |
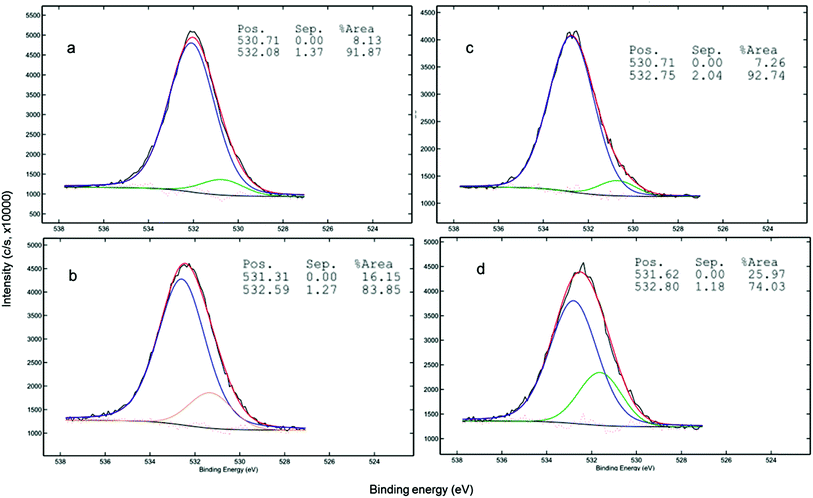
Evidence for surface complexation between the LDH-biochar composites and As can be found in XPS analysis of the As-loaded sorbents. Shifts in O1s peaks at 531.31 and 532.59 eV in NFMF, representing O bonded to metals and hydroxyl group bonded to metal and to H2O, respectively to 530.71 eV and 532.08 eV NFMF, respectively, suggest the reconfiguration of these bonds due to As complexation (Fig. 7). Similarly, NFMB peaks at 531.62 to 532.80 eV, for NFMB, shifted to 530.71 and 532.75 eV after As(V) sorption.
4. Concluding remarks
Two synthesis methods, pyrolysis of Ni/Fe-treated biomass and precipitation of Ni/Fe onto pristine biochars, produced carbonaceous sorbents with LDH as the dominant crystallite. The LDHs were generally evenly dispersed on the biochars surfaces, and XRD diffraction patterns indicated a layered Ni/Fe-LDH structure. The As(V) sorption capacity of NFMB at pH 7.5 was 4.38 g kg−1, roughly three times that by NFMF. The NFMF showed higher As(V) sorption capacity than some other Fe oxides modified biochars14,30 and have better performance at higher pH. The Ni/Fe-LDHs were the dominant sites of As(V) sorption, which occurred mainly through physisorption (electrostatic attraction and anion exchange) and formation of inner-sphere surface complexes. Its high sorption and the good desorption ability and high efficiency of regeneration make LDH-biochar composites a new class of sorbents with great potential for the environmental remediation of As(V) contamination in soils or aqueous solutions.Conflict of interest
The authors have declared no conflict of interest.Acknowledgements
This research was partially supported by the NSF (CBET-1054405) and NSFC (21428702).References
- S. Wang, B. Gao, A. R. Zimmerman, Y. Li, L. Ma, W. G. Harris and K. W. Migliaccio, Bioresour. Technol., 2015, 175, 391–395 CrossRef CAS PubMed.
- S. Zhang, H. Niu, Y. Cai, X. Zhao and Y. Shi, Chem. Eng. J., 2010, 158, 599–607 CrossRef CAS.
- L. Yang, M. Dadwhal, Z. Shahrivari, M. Ostwal, P. K. T. Liu, M. Sahimi and T. T. Tsotsis, Ind. Eng. Chem. Res., 2006, 45, 4742–4751 CrossRef CAS.
- I. Richardson, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2013, 69, 629–633 CrossRef CAS PubMed.
- M. Chetia, R. Goswamee, S. Banerjee, S. Chatterjee, L. Singh, R. Srivastava and H. Sarma, Clean Technol. Environ. Policy, 2012, 14, 21–27 CrossRef CAS.
- K.-H. Goh, T.-T. Lim and Z. Dong, Environ. Sci. Technol., 2009, 43, 2537–2543 CrossRef CAS PubMed.
- F. Li and X. Duan, in Layered Double Hydroxides, ed. X. Duan and D. Evans, Springer, Berlin Heidelberg, 2006, vol. 119, ch. 7, pp. 193–223 Search PubMed.
- X.-Y. Yu, T. Luo, Y. Jia, R.-X. Xu, C. Gao, Y.-X. Zhang, J.-H. Liu and X.-J. Huang, Nanoscale, 2012, 4, 3466–3474 RSC.
- P.-P. Huang, C.-Y. Cao, F. Wei, Y.-B. Sun and W.-G. Song, RSC Adv., 2015, 5, 10412–10417 RSC.
- Y. Yao, B. Gao, M. Inyang, A. R. Zimmerman, X. D. Cao, P. Pullammanappallil and L. Y. Yang, Bioresour. Technol., 2011, 102, 6273–6278 CrossRef CAS PubMed.
- D. Kang, X. Yu, S. Tong, M. Ge, J. Zuo, C. Cao and W. Song, Chem. Eng. J., 2013, 228, 731–740 CrossRef CAS.
- Y. Guo, Z. Zhu, Y. Qiu and J. Zhao, J. Environ. Sci., 2013, 25, 944–953 CrossRef CAS.
- A. Nakahira, T. Kubo and H. Murase, IEEE Trans. Magn., 2007, 43, 2442–2444 CrossRef CAS.
- M. Zhang, B. Gao, S. Varnoosfaderani, A. Hebard, Y. Yao and M. Inyang, Bioresour. Technol., 2013, 130, 457–462 CrossRef CAS PubMed.
- S. Wang, B. Gao, Y. Li, A. Mosa, A. R. Zimmerman, L. Q. Ma, W. G. Harris and K. W. Migliaccio, Bioresour. Technol., 2015, 181, 13–17 CrossRef CAS PubMed.
- J. Yan, L. Han, W. Gao, S. Xue and M. Chen, Bioresour. Technol., 2015, 175, 269–274 CrossRef CAS PubMed.
- M. Zhang and B. Gao, Chem. Eng. J., 2013, 226, 286–292 CrossRef CAS.
- M. Zhang, B. Gao, Y. Yao and M. Inyang, Chemosphere, 2013, 92, 1042–1047 CrossRef CAS PubMed.
- M. Zhang, B. Gao, J. Fang, A. E. Creamer and J. L. Ullman, RSC Adv., 2014, 4, 28171–28175 RSC.
- K. Hammes, R. J. Smernik, J. O. Skjemstad and M. W. I. Schmidt, Appl. Geochem., 2008, 23, 2113–2122 CrossRef CAS.
- S. C. Peterson, M. Appell, M. A. Jackson and A. A. Boateng, J. Agric. Sci., 2013, 5, 1–8 Search PubMed.
- M. Gong, Y. Li, H. Wang, Y. Liang, J. Z. Wu, J. Zhou, J. Wang, T. Regier, F. Wei and H. Dai, J. Am. Chem. Soc., 2013, 135, 8452–8455 CrossRef CAS PubMed.
- H. Sim, C. Jo, T. Yu, E. Lim, S. Yoon, J. H. Lee, J. Yoo, J. Lee and B. Lim, Chem.–Eur. J., 2014, 20, 14880–14884 CrossRef CAS PubMed.
- I. Langmuir, J. Am. Chem. Soc., 1916, 38, 2221–2295 CrossRef CAS.
- A. S. Ozcan, B. Erdem and A. Ozcan, Colloids Surf., A, 2005, 266, 73–81 CrossRef.
- X. Hu, Z. Ding, A. R. Zimmerman, S. Wang and B. Gao, Water Res., 2015, 68, 206–216 CrossRef CAS PubMed.
- S. Kumar, R. R. Nair, P. B. Pillai, S. N. Gupta, M. A. R. Iyengar and A. K. Sood, ACS Appl. Mater. Interfaces, 2014, 6, 17426–17436 CAS.
- S. Han, W. Hou, C. Zhang, D. Sun, X. Huang and A. Gouting Wang, J. Chem. Soc., Faraday Trans., 1998, 94, 915–918 RSC.
- S. Fendorf, M. J. Eick, P. Grossl and D. L. Sparks, Environ. Sci. Technol., 1997, 31, 315–320 CrossRef CAS.
- S. Wang, B. Gao, A. R. Zimmerman, Y. Li, L. Ma, W. G. Harris and K. W. Migliaccio, Bioresour. Technol., 2015, 175, 391–395 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra17490b |
| This journal is © The Royal Society of Chemistry 2016 |