In vitro and in vivo study of a colon-targeting resin microcapsule loading a novel prodrug, 3,4,5-tributyryl shikimic acid
Abstract
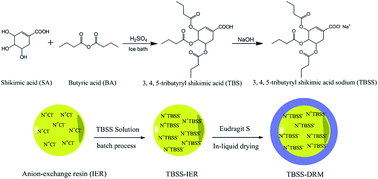
Prodrugs synthesized by different drugs not only overcome the defects of the original drugs, but also significantly enhance their treatment effects. In this study, a novel prodrug, 3,4,5-tributyryl shikimic acid (TBS), for the treatment of ulcerative colitis (UC) was synthesized by shikimic acid (SA) and butyric acid (BA) through the esterification reaction. Furthermore, the anion exchange resin, Amberlite 717 was employed to load the sodium salt of TBS through a batch process. Then the drug-loaded exchange resin (TBSS-IER) was encapsulated in the coating material, Eudragit S100, to prepare the colon-targeting drug resin microcapsule (TBSS-DRM) through an in-liquid drying method. The morphology and structure of TBSS-IER and TBSS-DRM were characterized by scanning electron microscopy (SEM). The in vitro release study demonstrated the good colon-targeting of TBSS-DRM. In the in vivo study, the TBSS-DRM exhibited a good therapeutic effect on the experimental colitis mouse induced by 2,4,6-trinitrobenzenesulfonic acid (TNBS). All results indicated that the prodrug was effective for colitis and the resin microcapsule system had good colon-targeting and could be used for the development of colon-targeting preparations.

 Please wait while we load your content...
Please wait while we load your content...