Selective recognition of lithium(I) ions using Biginelli based fluorescent organic nanoparticles in an aqueous medium†
Gaganpreet Kaura,
Amanpreet Singhb,
P. Venugopalanc,
Navneet Kaur*ac and
Narinder Singh*b
aCentre for Nanoscience & Nanotechnology (UIEAST), Panjab University, Chandigarh, 160014, India. E-mail: navneetkaur@pu.ac.in; Tel: +91-1722534464
bDepartment of Chemistry, Indian Institute of Technology Ropar, Rupnagar, Punjab, India 140001. E-mail: nsingh@iitrpr.ac.in; Fax: +91-1881223395; Tel: +91-1881242176
cDepartment of Chemistry, Panjab University, Chandigarh, 160014, India. E-mail: navneetkaur@pu.ac.in
First published on 18th December 2015
Abstract
This study presents the fabrication of a Biginelli compound 1 into fluorescent organic nanoparticles (ONP) using a reprecipitation method. The fabricated ONP were characterized by TEM and DLS. Further, the ONP were found to be selective towards Li(I) ions with a “fluorescence turn-on” mechanism and exhibited no significant response to possibly interfering cations (Na+, K+, Mg2+ and Ca2+) in a competitive experiment. The limit of detection was calculated to be 122 nM. It is apparent from the pH titration that the current sensor is independent of any variation in pH. To get a better insight of spacial arrangement, the crystal structure of 1 was solved by X-ray crystallography. The progress in sensing of lithium ions by various types of sensors on different instruments has also been discussed briefly.
Introduction
The design of novel fluorescent metal ions and molecular sensors based on organic nanoparticles is a dynamic area of research.1 Among the metal ions, lithium finds irrefutable industrial application in the development of lithium ion batteries and the recovery of lithium from spent batteries has also gained great economic interest.2 Lithium based medicines are routinely used in the treatment of manic depression and are potent therapeutic agents for the deficits caused by Traumatic Brain Injury (TBI).3 However, exposure to excessive lithium may result in detrimental effects to the nervous system, liver and brain.4 Consequently, its presence in environmental samples and human serum (allowed range: 0.6 and 1.2 mM) has to be monitored regularly. In this context, determination of lithium ions has received considerable attention and hence lithium sensing remains an imperative area of research. Analogous to other alkali metals, lithium exhibits poor coordination ability usually because of its smaller size and strong hydration in aqueous medium which makes it even more challenging to develop selective sensors for this purpose. However, its providentially high charge density (hard acid), in comparison to other alkali metals, enables it to successfully bind with the hard donor oxygen and nitrogen atoms present in the receptors.5 Another impediment in this pursuit lies in the fact that the presence of Na+, K+, Ca2+ and Mg2+ ions are known to interfere with the successful determination of lithium ions. In the initial stages of lithium detection, a variety of crown ethers, cryptands and other organic chromophores were fabricated for recognition of lithium ions.6 Unfortunately, these ligands possessed only weak binding properties in aqueous medium, since they are generally insoluble in water, thus limiting their application only in organic phases.Some of the commonly used techniques for determination of lithium ions are discussed here. Hamzaoui et al. developed a protocol for the quantitative determination of lithium traces in highly concentrated natural brines using flame spectrometry. They processed the samples by stepwise addition of BaCl2 and Na2CO3 to remove the SO42− and Mg2+ ions respectively, followed by addition of appropriate organic solvents in order to precipitate out other oceanic salts and prevent the interference phenomena.7 Nickus et al. derived the water residence time of a lake by analyzing it for the lithium ion content by application of ion exchange chromatography. To pursue this, a dilute LiCl solution was injected into the lake and the exponential decline of the lithium mass in the lake was studied.8 Gamiz-Gracia et al. proposed a method for the recognition of lithium ions in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water system using UV-Vis spectrophotometer and further applied it for detection of lithium in human serum and drugs, thus finding clinical application.9 Tabata et al. designed a porphyrin-based receptor for spectrophotometric lithium ion determination and used it to check the lithium levels in sea water and blood serum.10 Zawisza proposed an indirect method for determination of lithium through iron using X-ray fluorescence spectrometry and employed it for determining lithium values in ores.11 Kim et al. investigated the complex formation of azacrown indoaniline with Li(I) ions on a UV-Vis spectrophotometer.12 Other available techniques include thermal ionization mass spectrometry (TIMS),13 inductively coupled plasma atomic emission spectrometry (ICP-AES),14 differential pulse anodic stripping voltammetry (DPASV),15 capillary electrophoresis (CE),16 flow injection analysis (FIA)17 and ion chromatography (IC).18
water system using UV-Vis spectrophotometer and further applied it for detection of lithium in human serum and drugs, thus finding clinical application.9 Tabata et al. designed a porphyrin-based receptor for spectrophotometric lithium ion determination and used it to check the lithium levels in sea water and blood serum.10 Zawisza proposed an indirect method for determination of lithium through iron using X-ray fluorescence spectrometry and employed it for determining lithium values in ores.11 Kim et al. investigated the complex formation of azacrown indoaniline with Li(I) ions on a UV-Vis spectrophotometer.12 Other available techniques include thermal ionization mass spectrometry (TIMS),13 inductively coupled plasma atomic emission spectrometry (ICP-AES),14 differential pulse anodic stripping voltammetry (DPASV),15 capillary electrophoresis (CE),16 flow injection analysis (FIA)17 and ion chromatography (IC).18
Fluorescent sensing is known to proffer distinct advantages over most other instruments. For instance their ease of operation, rapid sensing, effortless maintenance, absence of reference electrodes and their great potential to be developed into portable instruments for point-of-care evaluation. Hence, a number of successful attempts have been made in designing of fluorescent sensors for lithium ion detection.19 Citterio et al. reported the synthesis of two novel lithium fluoroionophores containing 4-methylcoumarin as a fluorophore and 14-crown-4 as a Li(I)-binder which exhibited ICT type ratiometric fluorescence quenching along with a blue shift on lithium binding.20 Ando et al. designed a lithium sensing fluoroionophores based on boron-dipyrromethene as the fluorophore and immobilized it on a glass support and used the obtained optode to measure the Li(I) content in spiked human serum.21 Kumar et al. synthesized a fluorescent chemosensor based on thiacalix-[4]-crown which exhibited quenching of fluorescence intensity on addition of Fe3+ ions owing to ESIPT phenomenon. The receptor–Fe3+ complex thus obtained was further used as a secondary sensor for Li(I) ions via unquenching of fluorescence, attributed to the suppressing of PET.22 Kim et al. developed a fluorescence turn-on sensor by combining a squarylium dye (fluorophore) with a monoazacrown moiety (ionophore) which could effectively fit Li(I) ions in it.23 Grabchev et al. developed a dendrimer modified with naphthalimide units for the selective fluorescent sensing of Li(I) ions.24 Obare and his group has reported several fluorescent and UV-Vis lithium sensors some of which are based on 1,10 phenanthroline.25 Cametti et al. successfully recognized lithium ions by a salophen-UO2 homodimeric complex.26 Hiratani et al. developed an energy transfer fluorescent sensor in which the rotaxane unit acted as a host for the small lithium ions.27 Rochat et al. synthesized ruthenium based 12-metallacrown-3 complexes for selective detection of lithium in aqueous solution.28 The ligand developed by A. Caballero was based on ferrocene–anthracene-linked dyad which could sense lithium in aqueous environment.29
The proposed method offers a practical advantage for direct measurement of lithium ions in aqueous media in contrast to mostly available sensors. This has been achieved with the help of Biginelli-based organic nanoparticles, ONP (characterized by TEM and DLS). The ONP presented no response to interfering ions and exhibit sensing properties in a wide pH range. The selection of the Biginelli scaffold may be attributed to its straightforward one step synthesis and its selective sensing properties.30,31 This study may present new avenues for the sensing of lithium ions in aqueous medium using organic nanoparticles.
Result and discussion
Synthetic procedures
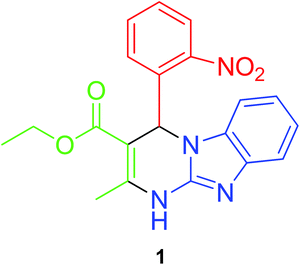
Synthesis of the Biginelli receptor (1) was carried out using our literature method30 which involves a single step, one pot cyclic condensation reaction between three reactants (Fig. 1). 2-Nitrobenzaldehyde (5 mmol), ethyl acetoacetate (5 mmol) and 2-aminobenzimidazole (5 mmol) were refluxed at 80 °C in methanol using zinc perchlorate hexahydrate as a catalyst. A yellow colour solid separated out on the completion of reaction. The solid product obtained was filtered, washed with methanol and dried before further use.X-ray structure description of 1
Fine crystals of 1 were obtained by its slow evaporation in acetonitrile solvent. The exact structure of the crystals thus obtained was investigated using single crystal X-ray analysis. The ORTEP plot and the atom numbering scheme is shown in Fig. 2. It is evident from the structure that the benzimidazole moiety has adhered to its usual planar nature. Furthermore, the formation of a hydrogen bond between the hydrogen atom of the chiral C–H and the oxygen of the NO2 group has also been established. It is quite apparent that this bonding has led to the formation of a 6-membered ring.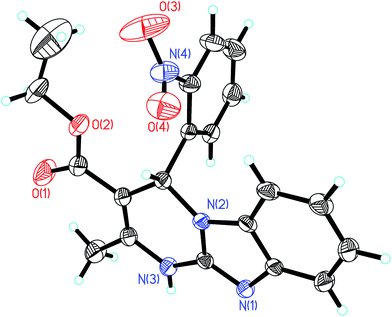 | ||
| Fig. 2 ORTEP diagram of compound 1 with 40% probability thermal ellipsoids and the atom-numbering scheme. | ||
Formation of organic nanoparticles (ONP)
The tremendous research interest in nanomaterials in the last decade can be accredited to its considerable relevance in nearly all possible streams like biological imaging, neuronal tissue engineering, and nucleic acid delivery.32–36 Organic nanoparticles in particular acquires interesting applications in pharmaceutical field as potential candidates for drug delivery. Further, nano biosensors have remarkably been used as optical sensors, electrochemical sensors, and mass sensitive sensors for the recognition of proteins, cancer cells and viruses. The key difference between organic and inorganic nanoparticles remains in their fabrication mechanism. Although inorganic nanoparticles are easily obtained from the precipitation of inorganic salts, organic nanoparticles are formed when several organic molecules congregate through chemical binding or self organization.Two common approaches usually used for the generation of organic nanoparticles are “top-down” (size reduction technique that involves complicated methods like mechanomilling and results in uneven size distribution) and “bottom-up” technique. “Bottom-up” method involves physicochemical processes like controlled assembly and self-organisation to build up nanomaterials from single molecules into larger structures. The bottom up approach consists of two methods: condensation and reprecipitation.
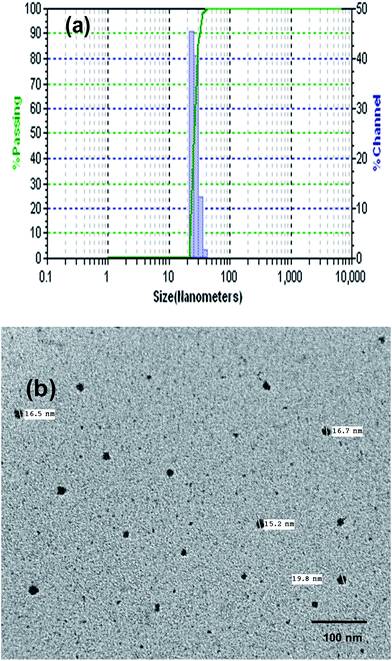
The reprecipitation method is a solvent displacement method in which the organic molecules are dissolved in appropriate solvents and gradually introduced in to the antisolvent (generally water) under continuous ultrasound treatment.37 The variation in the solubility of solvents is the motivating force for the generation of nanomaterials. The organic solution diffuses into the aqueous cores and penetrates inside after crossing the interfacial film. In the next stage, the organic molecules get precipitated due to their insolubility in water which induces nucleation of organic molecules. This is followed by solvent displacement and exchange of organic matter between the aqueous cores on collisions between different droplets. At last, the nuclei tend to grow in size. Several parameters that influence the size and shape of nanoparticles can be envisaged, for instance, nature of the organic compound involved, the selection of organic phase, aqueous phase, ratio of organic phase to aqueous phase, concentration, agitation speed and time and injection rate. In the present study, the organic compound 1 was dissolved in DMSO (1 mM). 0.5 mL of this organic solution was gradually injected into 100 mL water under continuous ultrasonication for about 15 minutes to form a 5 μM solution along with maintaining the temperature below 10 °C. The formation of ONP and their variation in size was investigated with the external probe feature of DLS (differential light scattering). DLS analysis revealed the average particle size of 25 nm (Fig. 3a). The polydispersity index from DLS measurements was found to be 0.06 with an average value of 25.2 and standard deviation of 6.1.
TEM analysis further confirmed the formation of discrete spherical nanoparticles of size 15–19 nm with an average value of 17 nm and std. deviation of 1.9 (Fig. 3b).
Solvent effect
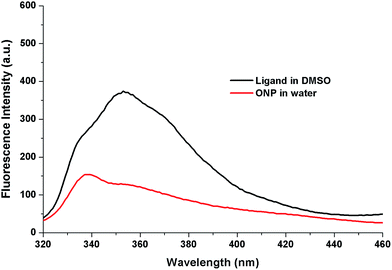
The influence of replacement of solvent from DMSO to water on the photo-physical properties of 1 was assessed using fluorescence spectrophotometer. To achieve this, the fluorescence emission spectra of compound 1 (5 μM) and its nanoparticles, ONP (5 μM) were recorded on excitation at 300 nm. Two interesting solvatochromic changes were perceived from the emission spectra on solvent modification. First is the decrease in the fluorescence intensity from 374 to 154 which may be attributed to “aggregation caused quenching” (ACQ) which occurs due to stacking interactions.38 Second is the hypsochromic (blue) shift from 353 nm to 339 nm (Fig. 4).The blue shift is generally obtained on the development of “H-type aggregates” where the molecules are arranged in a head to head or tail to tail fashion.39 The absorbance and excitation spectra of compound 1 (5 μM) in DMSO and its nanoparticles, ONP (5 μM) in water were also recorded which indicated changes in the profile on changing the solvent (Fig. S1†).
Recognition studies: cation binding with nanoparticles, ONP
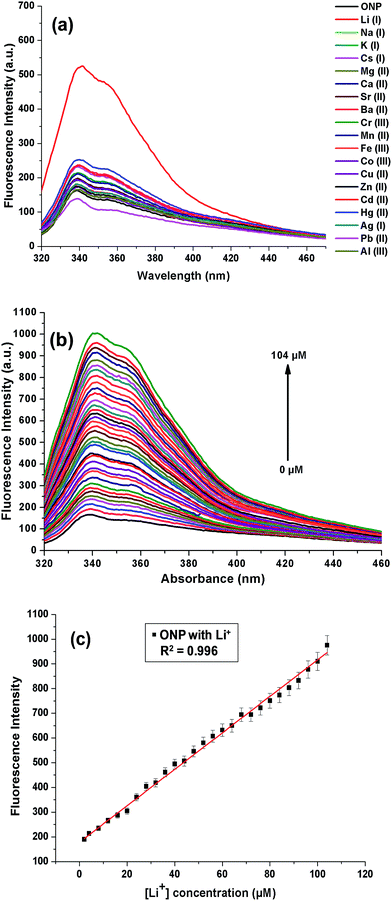
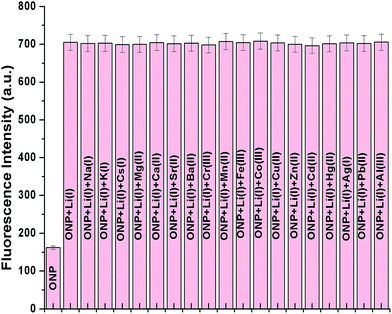
The cation binding ability of nanoparticles, ONP was assessed on a fluorescence spectrophotometer. This was pursued by recording changes in the emission profile of ONP on excitation at 300 nm. 50 μM metal nitrate solutions of different cations (Li+, Na+, K+, Cs+, Mg2+, Ca2+, Sr2+, Ba2+, Cr3+, Mn2+, Fe3+, Co2+, Cu2+, Zn2+, Hg2+, Pb2+, Cd2+, Ag+ and Al3+) were added to a fixed concentration of ONP (5 μM) taken in different 5 mL volumetric flasks. To ensure homogeneity of the solutions and successful binding with metal ions, all the solutions were well shaken and kept for 15 minutes before recording the fluorescence spectra. On addition of Li(I) ions, a substantial enhancement of fluorescence intensity was evident, although, no significant response could be accomplished with the other metal ions under study, thus projecting the organic nanoparticles, ONP as a selective sensor for Li(I) ions (Fig. 5a). The experiment was also repeated in HEPES buffered solution (pH 7.4), however, no marked change was observed which indicated that the ONP can be used even in the absence of any buffer (Fig. S2†). However, in solutions where a large pH change in expected, the use of HEPES buffer (pH 7.4) is encouraged.The augmentation in the fluorescence intensity may possibly be credited to strong interactions between the nanoparticles and Li(I) ions ensuing in a more static arrangement. A number of influences collectively may be responsible for the particular sensitivity of the sensor towards Li(I) ions. For instance, appropriate coordination site for small Li(I) ions or affinity of Li(I) ions towards highly electronegative oxygen and nitrogen atoms. Also, the presence of nitro group in compounds is often known to quench the fluorescence intensity. In the present case, it is possible that the Li(I) ions interact with the nitro group present on the 2-nitrophenyl moiety and results in the suppression of photoinduced electron transfer (PET).40 Once the binding of the receptor with Li(I) takes place, the nitro group is not freely available to quench the fluorescence, as a result of which the fluorescence was switched on after addition of Li(I) ions.
Further, the stability of ONP before and after addition of Li(I) was investigated over a period of time. The ONP once prepared were kept aside for a period one month and their fluorescence spectra was recorded (before and after addition of 50 μM Li(I)) after regular intervals of time (Fig. S3†). The absorbance spectra of ONP were also recorded over this time period. The graphs showed no remarkable change in the fluorescence intensity and absorbance which proves that nanoparticles were stable upto a period of 1 month.
This enhancement in the fluorescence intensity on addition of Li(I) to ONP was further explored by performing a titration where small aliquots of Li(I) ions were added into ONP and emission spectra were recorded (Fig. 5b). The increasing amount of Li(I) ion added to ONP was directly proportional to the enhancement of fluorescence intensity. Apparently, a good linearity response was observed from the results of the titration with an R2 value of 0.99625 (Fig. 5c). The detection limit of the current sensor for Li(I) ions in the solution was 122 nM, as calculated from the calibration plot using 3σ method. The absorbance and excitation spectra of ONP on addition of Li(I) was also recorded, which showed no change in the ONP profile (Fig. S4†). The binding of ONP with Li(I) was also established using DLS studies (Fig. S5†). It was found that the size of ONP increased on binding with Li(I) from 25 nm (ONP) to 30 nm (ONP + Li(I)).
Interference studies
The other group I and group II ions like Na+, K+, Mg2+ and Ca2+ are often known to interfere with the selective recognition of lithium ions. However, selectivity is an essential requisite for a superior sensor to work successfully in different environmental conditions even in presence of other analytes.Hence, in order to establish the ONP as a potential sensor for lithium ions, a competitive binding experiment was performed. To perform this study, the organic nanoparticles, ONP were taken in different 5 mL volumetric flasks and then 75 μM of lithium ion solution were added to each flask which resulted in the increase in fluorescence intensity. Further, all remaining metal solutions (Na+, K+, Cs+, Mg2+, Ca2+, Sr2+, Ba2+, Cr3+, Mn2+, Fe3+, Co2+, Cu2+, Zn2+, Hg2+, Pb2+, Cd2+, Ag+ and Al3+) were added in different volumetric flasks followed by recording fluorescence spectra for each solution. It is evident from the bar graph that the sensor is selective towards lithium ions and its sensing ability is not affected even in the presence of other interfering ions (Fig. 6). The experiment was also repeated in HEPES buffered solution (Fig. S6†), but, no remarkable change was observed when compared to Fig. 6. This implied the successful functioning of the sensor even in the absence of a buffer.
Response time, pH effect and salt effect
To determine the time taken by the ONP to bind with lithium ions, the response of fluorescence emission was determined as a function of time by investigating changes in the fluorescence spectra. In a typical experiment, varying concentrations of lithium ions (15, 30, 40 and 60 μM) were added to the ONP in four different volumetric flasks and fluorescence spectra were recorded at small intervals of time (Fig. 7). It is evident that lithium ions interacted with the ONP within the first 60 seconds of its addition and no remarkable change in the fluorescence spectra occurred thereafter. The practical utility of the sensor was further ascertained by examining its emission response at different pH values. Two different titrations were conducted to pursue this task. The acidic titration was performed by gradual addition of a dilute hydrochloric acid solution to the ONP and the basic titration was carried out using the sodium hydroxide solution. It can be perceived from the emission profiles that no considerable response could be determined between the pH range of 2–12 (Fig. S7†). Consequently, the proposed sensor may be employed for successful application in a broad pH range whether in the biomedical field or the battery industry. The stability in size of ONP on variation of pH was also investigated using DLS studies. The DLS histograms of ONP were recorded at varying pH and showed that the size of ONP remained almost same with changing pH (Fig. S8†). Salt effect studies were carried out by addition of 0–200 equivalent of tetrabutyl ammonium perchlorate salt. This experiment was performed to eliminate any chance of disturbance from high ionic strength on the action of sensor, (Fig. S9†). It was established that even under the influence of high salt concentration; no prominent changes in the fluorescence profile of sensor, thus removing any perturbation from high ionic strength.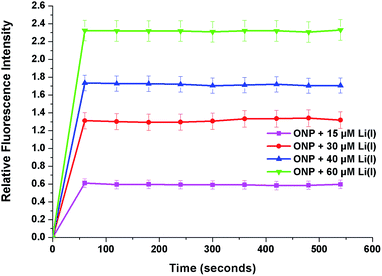 | ||
| Fig. 7 Plot of relative fluorescence intensity of ONP and Li(I) at different concentrations (15, 30, 40 and 60 μM) as a function of time (in seconds). | ||
Practical application in tap and lake water
To analyze the working capability of the sensor in practical samples, artificial samples were prepared. Tap water and lake water samples were collected. Any suspended impurities or biological material were removed using a syringe filter and then spiked with known concentration of Li(I) solution. The presence of lithium in these samples was then tested using the proposed method by recording their emission spectra and the results are presented in Table 1. It is apparent from the results that the sensor could successfully detect the presence of Li(I) in these practical samples with an average recovery percentage of 96.5%.Experimental
General information
The general information regarding the materials and instrumentation is given in ESI.† The CIF file for 1 was deposited in the Cambridge Structure Database with CCDC no. 1419344.†Synthesis
2-Nitrobenzaldehyde (5 mmol), 2-aminobenzimidazole (5 mmol) and ethyl acetoacetate (5 mmol) were dissolved together in 25 mL methanol in one pot and zinc perchlorate hexahydrate (0.5 mmol, 10 mol%) was used as a catalyst.30 The solution was heated under reflux at 80 °C with constant stirring until a yellow colored product separated out. The progress of the reaction was also monitored over silica coated TLC glass plates (chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol: 9.5
methanol: 9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 solvent system). After the completion of reaction, a yellow colour solid was collected, filtered and washed with methanol to get the final compound 1. Yellow solid; mp > 300 °C. 1H NMR (400 MHz, DMSO-d6) δ: 10.75 (brs, 1H, NH), 7.78 (t, J = 5.2 Hz, 1H, ArH), 7.43 (d, J = 7.8 Hz, 1H, ArH), 7.40 (d, J = 7.6 Hz, 1H, ArH), 7.35 (d, J = 7.9 Hz, 1H, ArH), 7.22 (d, J = 7.8 Hz, 1H, ArH), 7.15 (s, 1H, CH), 7.02 (t, J = 8.0 Hz, 1H, ArH), 6.90 (t, J = 8.0 Hz, 1H, ArH), 4.03 (m, 1H, CH2), 3.91 (m, 1H, CH2), 2.42 (s, 3H, CH3), 1.08 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 164.8, 147.7, 147.3, 145.3, 142.1, 136.8, 134.2, 131.7, 129.1, 128.9, 123.9, 122.2, 120.7, 117.0, 109.5, 97.3, 59.6, 50.6, 18.9, 14.0; CHN analysis calculated for (C20H18N4O4): C, 63.48; H, 4.79; N, 14.81, found: C, 64.03; H, 4.71; N, 14.24.
0.5 solvent system). After the completion of reaction, a yellow colour solid was collected, filtered and washed with methanol to get the final compound 1. Yellow solid; mp > 300 °C. 1H NMR (400 MHz, DMSO-d6) δ: 10.75 (brs, 1H, NH), 7.78 (t, J = 5.2 Hz, 1H, ArH), 7.43 (d, J = 7.8 Hz, 1H, ArH), 7.40 (d, J = 7.6 Hz, 1H, ArH), 7.35 (d, J = 7.9 Hz, 1H, ArH), 7.22 (d, J = 7.8 Hz, 1H, ArH), 7.15 (s, 1H, CH), 7.02 (t, J = 8.0 Hz, 1H, ArH), 6.90 (t, J = 8.0 Hz, 1H, ArH), 4.03 (m, 1H, CH2), 3.91 (m, 1H, CH2), 2.42 (s, 3H, CH3), 1.08 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 164.8, 147.7, 147.3, 145.3, 142.1, 136.8, 134.2, 131.7, 129.1, 128.9, 123.9, 122.2, 120.7, 117.0, 109.5, 97.3, 59.6, 50.6, 18.9, 14.0; CHN analysis calculated for (C20H18N4O4): C, 63.48; H, 4.79; N, 14.81, found: C, 64.03; H, 4.71; N, 14.24.
Production of organic nanoparticles, ONP
The basic single step re-precipitation method was used for synthesis of nanoparticles as this method proffers outstanding reproducibility, ease of operation and is quite economic. A (1 mM) solution of Biginelli compound 1 was prepared by dissolving 1 in 10 mL dimethyl sulfoxide solvent. 0.5 mL of this solution was gradually injected into 100 mL of water taken in a beaker with the help of an injection under continuous sonication for about 15 minutes to obtain the nanoparticles. Throughout the process, the temperature of the solution was maintained below 10 °C. This process was repeated several times and the size of formed nanoparticles was monitored by DLS probe to check for the reproducibility of results and optimization of parameters like time for sonication, injection rate etc.Recognition studies
All the recognition studies were performed at 25 ± 1 °C in deionized water. To assure the uniformity of solutions, the solutions were shaken and mixed for an adequate time and appropriately sonicated before conducting any fluorescence experiments. The binding capability of nanoparticles ONP (5 μM) against cations was investigated by adding 50 μM of different metal nitrate salts to 5 mL of ONP in different volumetric flasks. For titrations of ONP with Li(I) ions, LiNO3 solution was added to the ONP in small lots to a 5 mL volumetric flask containing nanoparticles, ONP. This was followed by recording of fluorescence spectra after each addition. For competitive binding experiment, Li(I) ion solution was added to the ONP in 19 different volumetric flasks. Once the fluorescence intensity of all the solution increased, the remaining cations under study were added to 18 different volumetric flasks at a concentration of 50 μM. The absence of any changes eliminates any probability of interference in the presence of other metal ions, especially Na+, K+, Ca2+ and Mg2+ ions.To investigate the effect of ionic strength on the practical applicability of sensor, different concentrations (0–200 equivalents) of tetrabutylammonium perchlorate salt were added to 10 mL solution of ONP taken in a volumetric flask, the fluorescence spectra were recorded after each addition. To confirm the action of nanoparticles on change in pH, pH titrations were also performed. 20 mL of ONP were taken in a beaker and a dilute solution of hydrochloric acid (0.1 mM) was added dropwise for varying the pH and performing acidic titration. Likewise, the basic titration was carried out using a dilute sodium hydroxide solution (0.1 mM) to change the pH in basic medium. There was not much change in the fluorescence emission profile of ONP on changing the pH from 2 to 12. For the real sample analysis, the collected water samples from tap (lab supply) and lake were prefiltered for removal of any visible turbidity.
Conclusions
The design and development of lithium ion sensors is a vigorous area of research owing to their demand for monitoring and understanding ion transport in lithium ion batteries and biomedical environments. We have described a new fluorescent organic nanoparticle based optical sensor suitable for measurement of lithium ions in aqueous media. The optical sensor is based on a Biginelli compound which has been fabricated into ONP via bottom up technique. The proposed nano sensor perceived no interference from any of the possible interfering cations and provided selective, rapid and stable response towards lithium ions. Further, the crystals of the Biginelli compound were analyzed by single crystal X-ray analysis which provided a better understanding of the structure of the ligand.Acknowledgements
GK, AS acknowledges CSIR, India for the JRF fellowship.Notes and references
- (a) A. Saini, J. Singh, R. Kaur, N. Singh and N. Kaur, New J. Chem., 2014, 38, 4580 RSC; (b) S. Kaur, A. Kaur, N. Kaur and N. Singh, Org. Biomol. Chem., 2014, 12, 8230 RSC; (c) S. Chopra, N. Singh, P. Thangarasu, V. K. Bhardwaj and N. Kaur, Dyes Pigm., 2014, 106, 45 CrossRef CAS.
- (a) X. Luo, B. Guo, J. Luo, F. Deng, S. Zhang, S. Luo and J. Crittenden, ACS Sustainable Chem. Eng., 2015, 3, 460 CrossRef CAS; (b) D. H. P. Kang, M. Chen and O. A. Ogunseitan, Environ. Sci. Technol., 2013, 47, 5495 CrossRef CAS PubMed.
- (a) P. R. Leeds, F. Yu, Z. Wang, C.-T. Chiu, Y. Zhang, Y. Leng, G. R. Linares and D.-M. Chuang, ACS Chem. Neurosci., 2014, 5, 422 CrossRef CAS PubMed; (b) J. K. Rybakowski, ACS Chem. Neurosci., 2014, 5, 413 CrossRef CAS PubMed.
- (a) R. F. McKnight, M. Adida, K. Budge, S. Stockton, G. M. Goodwin and J. R. Geddes, Lancet, 2012, 379, 721 CrossRef CAS PubMed; (b) A. Ivkovic and T. A. Stern, Psychosomatics, 2014, 55, 296 CrossRef PubMed; (c) R. Oruch, M. A. Elderbi, H. A. Khattab, I. F. Pryme and A. Lund, Eur. J. Pharmacol., 2014, 740, 464 CrossRef CAS PubMed; (d) A. Can, T. G. Schulze and T. D. Gould, Pharmacol., Biochem. Behav., 2014, 123, 3 CrossRef CAS PubMed.
- R. G. Pearson, Hard and Soft Acids and Bases, Dowden Hutchingson and Ross, Stroudsburg, PA, 1973 Search PubMed.
- (a) A. F. Sholl and I. O. Sutherland, J. Chem. Soc., Chem. Commun., 1992, 1716 RSC; (b) H. Sugihara and K. Hirantani, J. Synth. Org. Chem., Jpn., 1994, 52, 530 CrossRef CAS; (c) H. Sugihara, T. Okada and K. Hiratani, Anal. Sci., 1993, 9, 593 CrossRef CAS; (d) K. Hiratani, M. Nomoto, H. Sugihara and T. Okada, Analyst, 1992, 117, 1491 RSC; (e) K. Nakashima, S. Nakatsuji and S. Akiyama, Talanta, 1984, 31, 749 CrossRef CAS PubMed; (f) H. Nishida, Y. Katayama, H. Katsuki, M. Nakamuru, M. Takagi and K. Ueno, Chem. Lett., 1982, 1853 CrossRef CAS; (g) K. Kimura, S. Iketani and T. Shono, Anal. Chim. Acta, 1987, 203, 85 CrossRef CAS; (h) L. C. Rodríguez, C. J. Linares and M. R. F. Ceba, J. Anal. Chem., 1996, 356, 320 CrossRef PubMed; (i) K. Hiratani, Analyst, 1988, 113, 1065 RSC.
- H. Hamzaoui, A. M'nif and R. Rokbani, Talanta, 2006, 70, 847 CrossRef CAS PubMed.
- U. Nickus and H. Thies, J. Chromatogr. A, 2001, 920, 201 CrossRef CAS PubMed.
- L. G. Gracia, L. C. Rodriguez and M. R. Ceba, Talanta, 1997, 44, 75 CrossRef CAS PubMed.
- M. Tabata, J. Nishimoto and T. Kusano, Talanta, 1998, 46, 703 CrossRef CAS PubMed.
- B. Zawisza, J. Anal. At. Spectrom., 2010, 25, 34 RSC.
- S.-H. Kim, J.-W. Kim, J.-H. Kim, K. N. Koh and S.-W. Kang, Dyes Pigm., 2000, 46, 49 CrossRef CAS.
- N. Jabeen, E. Rehman and S. Ahmed, J. Radioanal. Nucl. Chem., 2003, 258, 427 CrossRef CAS.
- P. Lefton, R. Plaquet, F. Rose, G. Hennon and N. Ledeme, Anal. Chim. Acta, 1996, 327, 301 CrossRef.
- M. F. S. Teixeira, M. F. Bergamini and N. Bocchio, Talanta, 2004, 62, 603 CrossRef CAS PubMed.
- H. Okamoto, Y. Okamoto, T. Hirokawa and A. R. Timerbaev, Analyst, 2003, 128, 1439 RSC.
- (a) M. Cretin, L. Alerm, J. Bartroli and P. Fabry, Anal. Chim. Acta, 1997, 350, 7 CrossRef CAS; (b) C. M. L. da Silva, V. G. K. Almeida and R. J. Cassella, Talanta, 2007, 73, 613 CrossRef CAS PubMed.
- O. Zerbinati, F. Balduzzi and V. Dell'Orob, J. Chromatogr. A, 2000, 881, 645 CrossRef CAS PubMed.
- (a) R. Kataky, P. E. Nicholson and D. Parker, Analyst, 1991, 116, 135 RSC; (b) R. Kataky, P. E. Nicholson and D. Parker, J. Chem. Soc., Perkin Trans. 2, 1990, 321 RSC.
- D. Citterio, J. Takeda, M. Kosugi, H. Hisamoto, S. Sasaki, H. Komatsu and K. Suzuki, Anal. Chem., 2007, 79, 1237 CrossRef CAS PubMed.
- Y. Ando, Y. Hiruta, D. Citterio and K. Suzuki, Analyst, 2009, 134, 2314 RSC.
- M. Kumar, N. Kumar and V. Bhalla, Dalton Trans., 2013, 42, 981 RSC.
- S.-H. Kim, S.-K. Hana, S.-H. Park, C.-M. Yoon and S.-R. Keum, Dyes Pigm., 1999, 43, 21 CrossRef CAS.
- I. Grabchev, S. Dumas and J.-M. Chovelon, Dyes Pigm., 2009, 82, 336 CrossRef CAS.
- (a) S. O. Obare and C. J. Murphy, Inorg. Chem., 2001, 40, 6080 CrossRef CAS PubMed; (b) S. O. Obare, R. E. Hollowell and C. J. Murphy, Langmuir, 2002, 18, 10407 CrossRef CAS; (c) S. O. Obare and C. J. Murphy, New J. Chem., 2001, 25, 1600 RSC; (d) W. Qin, S. O. Obare, C. J. Murphy and S. M. Angel, Analyst, 2001, 126, 1499 RSC; (e) W. Qin, S. O. Obare, C. J. Murphy and S. M. Angel, Anal. Chem., 2002, 74, 4757 CrossRef CAS PubMed.
- M. Cametti, L. Ilander and K. Rissanen, Inorg. Chem., 2009, 48, 8632 CrossRef CAS PubMed.
- K. Hiratani, M. Kaneyama, Y. Nagawa, E. Koyama and M. Kanesato, J. Am. Chem. Soc., 2004, 126, 13568 CrossRef CAS PubMed.
- S. Rochat, Z. Grote and K. Severin, Org. Biomol. Chem., 2009, 7, 1147 CAS.
- A. Caballero, R. Tormos, A. Espinosa, M. D. Velasco, A. Tarraga, M. A. Miranda and P. Molina, Org. Lett., 2004, 24, 4599 CrossRef PubMed.
- N. Kaur, K. Kaur, T. Raj, G. Kaur, A. Singh, T. Aree, S. Park, T. Kim, N. Singh and D. O. Jang, Tetrahedron, 2015, 71, 332 CrossRef CAS.
- G. Kaur, T. Raj, N. Kaur and N. Singh, Org. Biomol. Chem., 2015, 13, 4673 CAS.
- J. M. Fuente and V. Grazu, Nanobiotechnology: Inorganic Nanoparticles vs. Organic Nanoparticles, 2012, vol. 4, ISBN: 978-0-12-415769-9 Search PubMed.
- G. Schmid, Nanoparticles: from theory to application, Wiley-VCH Publisher, Weinheim, 2004 Search PubMed.
- M. Hosokawa, K. Nogi, M. Naito and T. Yokoyama, Nanoparticle technology handbook, Elsevier, Amsterdam, 2007 Search PubMed.
- K. E. Geckeler and H. Nishide, Advanced nanomaterials, Wiley-VCH Publishers, Weinheim, 2010 Search PubMed.
- J. Allouche, Synthesis of organic and bioorganic nanoparticles: An overview of the preparation methods, 2013, ch. 2, ISBN: 978-1-4471-4212-6 Search PubMed.
- F. Debuigne, L. Jeunieau, M. Wiame and J. B. Nagy, Langmuir, 2000, 16, 7605 CrossRef CAS.
- Q. Zeng, Z. Li, Y. Dong, C. Di, A. Qin, Y. Hong, L. Ji, Z. Zhu, C. K. W. Jim, G. Yu, Q. Li, Z. Li, Y. Liu, J. Qin and B. Z. Tang, Chem. Commun., 2007, 70 RSC.
- A. Zitzler-Kunkel, M. R. Lenze, K. Meerholz and F. Würthner, Chem. Sci., 2013, 4, 207 RSC.
- (a) N. Wanichecheva, J. S. Benco, C. R. Lambert and W. G. McGimpsey, Photochem. Photobiol., 2006, 82, 268 CrossRef CAS PubMed; (b) J. S. Benco, H. A. Nienaber and W. G. McGimpsey, J. Photochem. Photobiol., A, 2004, 162, 289 CrossRef CAS; (c) T. Gunnlaugsson, B. Bichell and C. Nolan, Tetrahedron, 2004, 60, 5799 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fluorescence spectra, X-ray crystallography data. CCDC 1419344. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra16743d |
| This journal is © The Royal Society of Chemistry 2016 |