Investigation the interaction between protamine sulfate and CdTe quantum dots with spectroscopic techniques†
Fangfang Xue‡
,
Lingzhi Liu‡,
Yueyao Mi,
Heyou Han and
Jiangong Liang*
College of Science, State Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University, Wuhan 430070, China. E-mail: liangjg@mail.hzau.edu.cn; Fax: +86 27 8728 2133; Tel: +86 27 8728 3712
First published on 21st January 2016
Abstract
The interaction between protamine sulfate and CdTe quantum dots (QDs) was investigated in detail by spectroscopic techniques. It was found that the fluorescence of QDs was quenched intensely by protamine sulfate. According to the results of lifetime measurements, temperature dependence, UV-Vis absorption spectra, calorimetric titration and zeta potential measurements, a combined dynamic and static quenching model was proposed. The binding force was also discussed and the process was found to be associated with electrostatic interaction. The results of this work indicated that the charge of protein has great effect on the emission of QDs. Moreover, it should contribute to the studies of interactions of QDs with proteins and promotes its application in biological systems.
1. Introduction
Since Bawendi and coworkers synthesized highly luminescent quantum dots (QDs) in 1993,1 QDs have attracted great attention due to their enormous advantages over conventional organic dyes. As a novel fluorescent probe, QDs have been widely used in cellular imaging,2 single molecule detection,3 in vivo animal targeting4 and photodynamic therapy.5 A major problem of QDs as tracking molecules in bioimaging is their strong nonspecific adsorption of various cellular proteins, which may prevent QDs-ligands conjugates from interacting with their cellular receptors, even losing specificity.6 Therefore, understanding the interaction of QDs with proteins and its mechanism could provide new insights into QDs based biomedical applications.Up to now, many analytical techniques, including capillary electrophoresis (CE),7 resonance Rayleigh scattering (RRS),8 fluorescence correlation spectroscopy (FCS),9 dynamic light scattering (DLS),10 surface plasmon resonance (SPR)11,12 and atomic force microscopy (AFM)13 have been applied to study the interactions of QDs with proteins. Owing to the extreme sensitivity and versatility, spectroscopic techniques have been one of the most extensive methods. With this method, the interactions of QDs with human serum albumin (HSA),8,11,14 bovine serum albumin (BSA),9,13,15–19 hemoglobin (Hb),12 glucose oxidase (GOD),12 cytochrome c (Cyt C)12 etc., have been reported, and the interaction mechanisms have been investigated. However, these studies mainly focused on proteins with negative charge. Detailed investigations on interactions of QDs with positively charged proteins are rare.
Protamine sulfate isolated from salmon is a highly cationic polypeptide with molecular weight of approximately 5 kDa and is used commonly in clinical medicine. It has antibacterial properties20 and can neutralize the anticoagulant activity of heparin.21 Meanwhile, it can be used to bind DNA.22 The high arginine content (approximately 66%) of protamine sulfate forms a high isoelectric point of 12–13 and results in a very high positive charge under physiological buffers at pH 7.4.23 Although several methods for protamine detections based on nanomaterials aggregations have been reported recently,24–26 the detailed interaction mechanism of QDs with protamine sulfate is still of great interest and helps promote a better understanding of the interactions of QDs with positively charged proteins.
Herein in the present work, we used spectroscopic techniques, including fluorescence, UV-Vis and fluorescence lifetime to investigate the interaction of protamine sulfate with CdTe QDs. It was found that the fluorescence of CdTe QDs was strongly quenched by protamine sulfate. A combined dynamic and static quenching mechanism was proposed to explain the interaction. The binding force was also discussed on the basis of calorimetric titration, zeta potential measurements and size distribution analysis.
2. Experimental section
2.1 Reagents
Thioglycolic acid (TGA), CdCl2·2.5H2O, sodium tellurite (Na2TeO3), sodium borohydride (NaBH4) and trisodium citrate dihydrate were obtained from Sinopharm Chemical Reagent Co. (Shanghai, China). Protamine sulfate, purchased from Sigma (St. Louis, MO), was dissolved in ultrapure water at a concentration of 1.0 × 10−5 M and was kept at 4 °C for further use. All other chemicals were of analytical grade and were used without further purification. All the solutions were prepared in ultrapure water with a resistivity of 18.2 MΩ cm.2.2 Instruments
The UV-Vis absorption spectra were measured using a UV2450 UV-Vis spectrophotometer (Shimadzu Scientific Instruments Inc.) equipped with 1 cm quartz cell. The fluorescence spectra and fluorescence lifetime were recorded on a FLS 920 steady state and lifetime spectrofluorimeter (Edinburgh Instruments Ltd.) with the excitation of 390 nm. The zeta potential measurements and particle size distribution measurements were carried out on a Zetasizer Nano ZS instrument (Malvern Instruments Ltd.). The calorimetric titration of QDs by protamine sulfate was performed on a NANO ITC LV isothermal titration calorimeter (TA Instruments) at 298 K. High resolution transmission electron microscopy (HRTEM) image was acquired by a Tecnai G2 F30 transmission electron microscope (FEI Company) operating at an acceleration voltage of 300 kV. The circular dichroism (CD) spectra were recorded from 250 nm to 190 nm on a J-1500 CD spectrometer (JASCO Corporation). All the pH measurements were made with a Model pHS-3C meter (Shanghai REX Instrument Factory).2.3 Preparation and purification of TGA-capped CdTe QDs
Two different sized TGA-capped CdTe QDs were synthesized following the method reported previously with some modifications.27 Briefly, 0.0457 g (0.200 mmol) CdCl2·2.5H2O was dissolved in 100 mL of ultrapure water bubbled with N2 for 30 min. Subsequently, 20 μL of TGA (0.288 mmol) was added and the pH of the mixture was adjusted to 11.0 by dropwise addition of 1.0 M NaOH solution under N2 atmosphere. Then trisodium citrate dihydrate (0.1076 g, 0.366 mmol), Na2TeO3 (0.0088 g, 0.0397 mmol), and NaBH4 (0.0048 g, 0.127 mmol) were added into the above mixture, followed by refluxing at 100 °C for different times to control the particle sizes. The crude products were precipitated by acetone with centrifugation at 8000 rpm for 5 min and the resultant precipitate was redispersed in ultrapure water, then kept at 4 °C in dark for further use.The particles sizes of CdTe QDs were determined from the first absorption maximum of the UV-Vis absorption spectra according to the following equation:28
| D = (9.8127 × 10−7)λ3 − (1.7147 × 10−3)λ2 + (1.0064)λ − (194.84) | (1) |
2.4 Investigation of the interaction between CdTe QDs and protamine sulfate
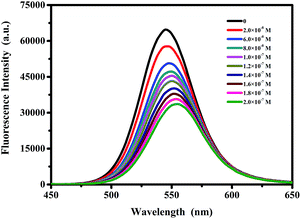
In order to investigate the effect of protamine sulfate on the fluorescence of CdTe QDs, varying volumes of 1.0 × 10−5 M protamine sulfate were allowed to interact with 4.0 × 10−7 M 545 nm QDs. After incubating for 1 h at room temperature with gentle shaking, the fluorescence emission of 545 nm QDs was measured under the excitation of 390 nm. The experiments were also conducted with 620 nm QDs for comparison.3. Results and discussion
3.1 Photophysical properties of QDs with different sizes
The absorption and fluorescence properties of the two different sized TGA-CdTe QDs were characterized first. As shown in Fig. 1, the maximum absorption of 545 nm and 620 nm CdTe QDs locate at 495 nm and 570 nm, respectively, indicating the effect of quantum confinement.1 The fluorescence emission spectra of the two CdTe QDs are both narrow and symmetric, indicating their good size distribution. With rhodamine 6G as the reference,29 the fluorescence quantum yields of 545 nm and 620 nm CdTe QDs were determined to be 24.10% ± 0.87% and 6.79% ± 0.41%, respectively.The particles sizes of CdTe QDs could be controlled simply by varying the reaction time and were determined by eqn (1). The results showed that the particle sizes of the as-prepared CdTe QDs were around 2.2 nm and 3.4 nm, corresponding with the first absorption maximum of 495 nm and 570 nm. And their concentrations were calculated from its absorption using Lambert–Beer's law:
| A = εcl | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 043(D)2.12,28
043(D)2.12,28
The morphology and size of the as-prepared 545 nm CdTe QDs were also characterized in detail by HRTEM and DLS analysis. HRTEM image (ESI Fig. S1a†) showed that the synthesized QDs are well dispersed and uniform in shape. The existence of well-resolved lattice planes indicated the excellent crystalline structures of the QDs. The mean particle size of the QDs was determined to be 3.0 ± 0.7 nm. The DLS measurement (ESI Fig. S1b†) revealed that the particles have good size distribution with an average size of 9 nm, larger than the sizes measured by HRTEM and UV-Vis absorption spectrum.
3.2 The effect of protamine sulfate on the fluorescence of CdTe QDs
In order to study the interaction between protamine sulfate and CdTe QDs, the effect of protamine sulfate on the fluorescence of 545 nm CdTe QDs was investigated. Typically, different amounts of protamine sulfate were added to a fixed concentration of QDs solutions (4.0 × 10−7 M in 10 mM phosphate buffered saline, pH 7.4). The incubation time was explored and was selected as 1 h according to the results of ESI Fig. S2.† With increasing amounts of protamine sulfate, the fluorescence intensity of QDs decreased gradually, indicating an interaction between protamine sulfate and QDs (Fig. 2). Moreover, the maximum emission of QDs exhibits a remarkable red shift from 545 nm to 552 nm with the addition of protamine sulfate, suggesting that the surface state of QDs may be changed under the interaction of protamine sulfate.Generally, the quenching mechanism can be divided into either static quenching or dynamic quenching or a mixture of the two. No matter which quenching mechanism, the fluorescence quenching data can be analyzed by the Stern–Volmer equation,30
| F0/F = 1 + KSV[Q] | (3) |
3.3 Investigation of quenching mechanism and binding forces
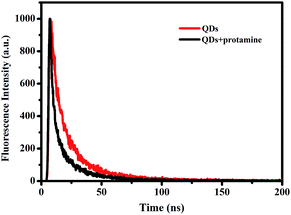
Fluorescence quenching can result from a variety of molecular interactions, including excited-state reactions, molecular rearrangements, energy transfer, ground-state complex formation, and collisional quenching.30 To understand the fluorescence quenching mechanism of QDs by protamine sulfate, UV-Vis absorption measurements, dependence on temperature and fluorescence lifetime experiments were conducted.| τ = (B1τ12 + B2τ22)/(B1τ1 + B2τ2) | (4) |
The fitting parameters of τ1, τ2, B1, B2, and τ (Fig. 4) are summarized in Table 1. From the data, it can be seen that there are two different lifetimes, which can be attributed to the intrinsic recombination of populated core states and the involvement of surface states respectively.33,34 With the addition of protamine sulfate, the average lifetime of CdTe QDs decreased from 24.96 ns to 19.70 ns as the longer lifetime decreased from 29.04 ns to 21.19 ns, implying the decrease of surface-related emission. The results indicated that the surface state of QDs has been changed as protamine sulfate was added. The decrease in fluorescence lifetime proved that the observed quenching is due to a dynamic quenching.
| Sample | B1 (%) | τ1 (ns) | B2 (%) | τ2 (ns) | τ (ns) |
|---|---|---|---|---|---|
| CdTe QDs | 45.93 ± 2.43 | 8.38 ± 0.40 | 54.07 ± 2.43 | 29.04 ± 1.11 | 24.96 ± 0.62 |
| CdTe QDs with protamine sulfate | 35.57 ± 2.12 | 3.53 ± 0.37 | 64.43 ± 2.12 | 21.19 ± 1.23 | 19.70 ± 1.02 |
 | ||
| Fig. 5 Stern–Volmer plots for the quenching of 545 nm QDs by protamine sulfate at different temperatures under pH 7.4. | ||
In general, four binding forces, i.e., hydrogen bonding, Van der Waals interaction, hydrophobic force and electrostatic attraction are usually used to explain the interactions between QDs and macromolecules.14 The type of binding forces can be distinguished by their differing thermodynamic parameters. Isothermal titration calorimetry (ITC) is a good technique to measure the binding affinity constant, free energy changes (ΔG), enthalpy changes (ΔH), entropy changes (ΔS) and binding stoichiometry of the interaction between nanoparticles and proteins.35 Herein, the thermodynamic parameters of the interaction were obtained by ITC. On the basis of the results (ESI Fig. S3†), ΔG, ΔH and ΔS were observed to be −34.28 kJ mol−1, −20.01 kJ mol−1 and 47.89 J mol−1 K−1, respectively.
The negative value of ΔG and negative value of ΔH revealed that the interaction process is spontaneous and exothermic. The positive entropy change ΔS showed that the surrounding water molecules acquired a more random configuration. Considering the negative enthalpy change and positive entropy change, the process was associated with electrostatic interaction.36
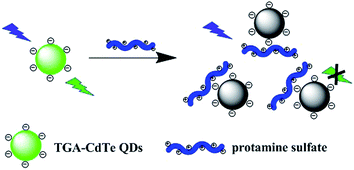
The results of lifetime measurements, temperature dependence and absorption spectra showed that the quenching of 545 nm QDs by protamine sulfate is mainly initiated by a dynamic process. However, the quenching rate constant Kq, calculated from the equation Kq = KSV/τ0, was found to be 1.67 × 1014 M−1 s−1 for 545 nm QDs, several orders of magnitude greater than the maximum value for diffusion-controlled quenching (1010 M−1 s−1).37 Considering the reported effect of protamine on nanomaterial aggregation,24–26,38 a possible quenching mechanism was proposed as depicted in Scheme 1. The high arginine content makes protamine sulfate a high isoelectric point and results in a high positive charge at pH 7.4. The capping reagent TGA on the surface of CdTe QDs makes it negatively charged at pH 7.4. Upon mixing, the electrostatic interaction between positive charge and negative charge would bring protamine sulfate and QDs in proximity and form complex. Meanwhile, protamine sulfate will collide with the excited QDs. Thus, a combined dynamic and static quenching was happened.
To further support this hypothesis, zeta potential measurement and size distribution analysis were carried out. The zeta potential of the as-prepared TGA modified 545 nm QDs was detected to be −19.3 mV, confirming that QDs was negatively charged at pH 7.4. And, it was changed to 19.8 mV after interact with protamine sulfate, further confirming the interaction of QDs with protein. Lu et al.12 have studied the interaction of negatively charged QDs with proteins possessing different isoelectric points. The results indicated that the surface charge distribution play a significant role in the protein interaction with QDs, since the interaction capability of positively charged proteins is higher than that of the negatively charged ones. The results of zeta potential was in accordance with the literature and suggested that the charge of protein plays a significant role in the interactions of QDs with proteins. In addition, the average hydrodynamic diameter of 545 nm QDs in aqueous solution is 9 nm. With the presence of protamine sulfate, it increased to 21 nm, indicating that static quenching also plays an important role. Lai et al.39 have found that the electrostatic interaction between positively coated QDs and negatively charged serum albumin could results in the aggregation of QDs. Therefore, the larger hydrodynamic diameter of QDs in the presence of protamine sulfate revealed that the electrostatic interaction between QDs and protamine induces the formation of small aggregates.
In the previous research, Wang et al.40 have investigated the interactions between BSA and CdTe QDs. The results have shown that BSA can enhance the fluorescence intensity of QDs. However, for the interaction of protamine sulfate with CdTe QDs, a fluorescence quench was observed. At pH 7.4, BSA is negatively charged, and protamine sulfate is positively charged. Therefore, it can be concluded that the charge of protein has great effect on the interactions of QDs with proteins. What's more, from the results of CD, it can be seen that the structure of protamine sulfate has been changed after addition of CdTe QDs (ESI Fig. S4†).
According to the above results, a combined dynamic and static quenching mechanism was proposed to explain the fluorescence quenching of 545 nm QDs by protamine sulfate. The interaction between protamine sulfate and 620 nm CdTe QDs was also investigated, and the same phenomenon was observed (ESI Fig. S5 and S6†). The Stern–Volmer quenching constant KSV was determined to be 8.94 × 106 M−1, higher than that of 545 nm QDs. The larger size of 620 nm QDs may enhance the affinity for protamine sulfate, and thus induce a stronger interaction. The results indicated that particle size plays an important role in the interaction of QDs with protein.
4. Conclusions
In this work, the interaction between protamine sulfate and CdTe QDs was investigated in detail with spectroscopic techniques. Upon mixing, the fluorescence of QDs was quenched remarkably by protamine sulfate. Fluorescence lifetime, dependence of temperature combined with UV-Vis absorption spectra were conducted to explore the quenching mechanism and the binding force was also discussed. A combined dynamic and static quenching mechanism was proposed. The results of this work suggest that the charge of protein has great effect on the interactions of QDs with proteins. Moreover, it provides new insights into the development of interactions between QDs and proteins and promotes its application in biological systems.Acknowledgements
Financial support from the National Natural Science Foundation of China (No. 21205043 and 31372439) is gratefully acknowledged.Notes and references
- C. B. Murray, D. J. Norris and M. G. Bawendi, J. Am. Chem. Soc., 1993, 115, 8706 CrossRef CAS.
- X. H. Gao, L. L. Yang, J. A. Petros, F. F. Marshall, J. W. Simons and S. M. Nie, Curr. Opin. Biotechnol., 2005, 16, 63 CrossRef CAS PubMed.
- Q. Ma and X. G. Su, Analyst, 2011, 136, 4883 RSC.
- X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538 CrossRef CAS PubMed.
- A. C. S. Samia, X. B. Chen and C. Burda, J. Am. Chem. Soc., 2003, 125, 15736 CrossRef CAS PubMed.
- M. M. Barroso, J. Histochem. Cytochem., 2011, 59, 237 CrossRef CAS PubMed.
- L. W. Shao, C. Q. Dong, X. Y. Huang and J. C. Ren, Chin. Chem. Lett., 2008, 19, 707 CrossRef CAS.
- K. J. Huang, C. Y. Wei, Y. M. Shi, W. Z. Xie and W. Wang, Spectrochim. Acta, Part A, 2010, 75, 1031 CrossRef PubMed.
- L. W. Shao, C. Q. Dong, F. M. Sang, H. F. Qian and J. C. Ren, J. Fluoresc., 2009, 19, 151 CrossRef CAS PubMed.
- B. I. Ipe, A. Shukla, H. C. Lu, B. Zou, H. Rehage and C. M. Niemeyer, ChemPhysChem, 2006, 7, 1112 CrossRef CAS PubMed.
- Q. Xiao, B. Zhou, S. Huang, F. F. Tian, H. L. Guan, Y. S. Ge, X. R. Liu, Z. K. He and Y. Liu, Nanotechnology, 2009, 20, 325101 CrossRef PubMed.
- Z. S. Lu, W. H. Hu, H. F. Bao, Y. Qiao and C. M. Li, Med. Chem. Commun., 2011, 2, 283 RSC.
- V. Poderys, M. Matulionyte, A. Selskis and R. Rotomskis, Nanoscale Res. Lett., 2011, 6, 9 Search PubMed.
- Q. Xiao, S. Huang, Z. D. Qi, B. Zhou, Z. K. He and Y. Liu, Biochim. Biophys. Acta, 2008, 1784, 1020 CrossRef CAS PubMed.
- J. G. Liang, Y. P. Cheng and H. Y. Han, J. Mol. Struct., 2008, 892, 116 CrossRef CAS.
- M. M. Dzagli, V. Canpean, M. Iosin, M. A. Mohou and S. Astilean, J. Photochem. Photobiol., A, 2010, 215, 118 CrossRef CAS.
- M. A. Jhonsi, A. Kathiravan and R. Renganathan, Colloids Surf., B, 2009, 72, 167 CrossRef PubMed.
- Q. S. Wang, X. L. Zhang, X. L. Zhou, T. T. Fang, P. F. Liu, P. Liu, X. M. Min and X. Li, J. Lumin., 2012, 132, 1695 CrossRef CAS.
- D. D. Wu, Z. Chen and X. G. Liu, Spectrochim. Acta, Part A, 2011, 84, 178 CrossRef CAS PubMed.
- A. Aspedon and E. A. Groisman, Microbiology, 1996, 142, 3389 CrossRef CAS PubMed.
- L. Falkon, M. Garí, I. Gich and J. Fontcuberta, Thromb. Res., 1998, 89, 79 CrossRef CAS PubMed.
- L. Willmitzer and K. G. Wagner, Biophys. Struct. Mech., 1980, 6, 95 CrossRef CAS PubMed.
- D. Awotwe-Otoo, Ph.D. Thesis, Howard University, 2011.
- X. Peng, Q. Long, H. T. Li, Y. Y. Zhang and S. Z. Yao, Spectrochim. Acta, Part B, 2015, 213, 131 CAS.
- A. A. Ensafi, N. Kazemifard and B. Rezaei, Biosens. Bioelectron., 2015, 71, 243 CrossRef CAS PubMed.
- Z. F. Zhang, Y. M. Miao, Q. D. Zhang and G. Q. Yan, Anal. Biochem., 2015, 478, 90 CrossRef CAS PubMed.
- Z. H. Sheng, H. Y. Han and J. G. Liang, Luminescence, 2009, 24, 271 CrossRef CAS PubMed.
- W. W. Yu, L. H. Qu, W. Z. Guo and X. G. Peng, Chem. Mater., 2003, 15, 2854 CrossRef CAS.
- M. Grabolle, M. Spieles, V. Lesnyak, N. Gaponik, A. Eychmüller and U. Resch-Genger, Anal. Chem., 2009, 81, 6285 CrossRef CAS.
- J. R. Lakowicz, Quenching of Fluorescence, in Principles of Fluorescence Spectroscopy, Springer Academic, New York, 2006, p. 277 Search PubMed.
- Y. Lu, M. Dasog, A. F. G. Leontowich, R. W. J. Scott and M. F. Paige, J. Phys. Chem. C, 2010, 114, 17446 CAS.
- P. Yang, M. Ando, T. Taguchi and N. Murase, J. Phys. Chem. C, 2010, 114, 20962 CAS.
- X. Y. Wang, L. H. Qu, J. Y. Zhang, X. G. Peng and M. Xiao, Nano Lett., 2003, 3, 1103 CrossRef CAS.
- K. Zhao, J. Li, H. Z. Wang, J. Q. Zhuang and W. S. Yang, J. Phys. Chem. C, 2007, 111, 5618 CAS.
- M. Mahmoudi, I. Lynch, M. R. Ejtehadi, M. P. Monopoli, F. B. Bombelli and S. Laurent, Chem. Rev., 2011, 111, 5610 CrossRef CAS PubMed.
- P. D. Ross and S. Subramanian, Biochemistry, 1981, 20, 3096 CrossRef CAS PubMed.
- Z. J. Cheng, R. Liu, X. H. Jiang and Q. Y. Xu, Luminescence, 2014, 29, 504 CrossRef CAS PubMed.
- S. N. Ding, C. M. Li and N. Bao, Biosens. Bioelectron., 2015, 64, 333 CrossRef CAS PubMed.
- L. Lai, C. Lin, Z. Q. Xu, X. L. Han, F. F. Tian, P. Mei, D. W. Li, Y. S. Ge, F. L. Jiang, Y. Z. Zhang and Y. Liu, Spectrochim. Acta, Part A, 2012, 97, 366 CrossRef CAS PubMed.
- H. P. Wang, C. Z. Zheng, T. J. Dong, K. L. Liu, H. Y. Han and J. G. Liang, J. Phys. Chem. C, 2013, 117, 3011 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: The effect of incubation time, the calorimetric titration of 545 nm CdTe QDs by protamine sulfate, the effect of protamine sulfate concentrations on fluorescence spectra of 620 nm QDs, and the fluorescence decay curves of 620 nm QDs in the absence and presence of protamine sulfate. See DOI: 10.1039/c5ra16586e |
| ‡ The first two authors F. F. Xue and L. Z. Liu have equal contributions to this work. |
| This journal is © The Royal Society of Chemistry 2016 |