Thiourea assisted hydrothermal synthesis of ZnS/CdS/Ag2S nanocatalysts for photocatalytic degradation of Congo red under direct sunlight illumination
Abstract
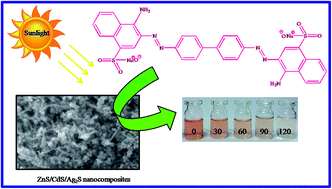
Solar light active ternary ZnS/CdS/Ag2S nanocatalysts have been synthesized via a hydrothermal process. The crystal structure, optical, morphological and surface area of the catalyst are characterized by XRD, UV DRS, PL, FE-SEM and BET measurements. The ternary catalyst has a decreased band gap energy value compared to that of the ZnS semiconductor. The catalyst was scrutinized for its catalytic performance under direct sunlight towards the degradation of a textile dye – Congo red at different time intervals. The results confirm that the Ag2S incorporated ternary ZnS/CdS/Ag2S nanocatalyst has good photocatalytic performance under direct sunlight when compared to binary and mono semiconductor systems.

 Please wait while we load your content...
Please wait while we load your content...