Dendrosome mediated topical gene silencing by PLK-1 specific siRNA: implication in treatment of skin cancer in mouse model
Mohd. Asif Sherwania,
Saba Tufaila,
Aijaz Ahmed Khan*b and
Mohammad Owais*a
aInterdisciplinary Biotechnology Unit, Aligarh Muslim University, Aligarh, 202002, India. E-mail: owais_lakhnawi@rediffmail.com; Fax: +91-571-2721776; Tel: +91-571-2720388
bDepartment of Anatomy, Jawaharlal Nehru Medical College, Faculty of Medicine, Aligarh Muslim University, Aligarh, 202002, India. E-mail: aijazahmedkhan7@live.com
First published on 11th January 2016
Abstract
Although topical application of nucleic acids offers many potential therapeutic advantages for suppressing genes in the skin, topical delivery of siRNA for skin cancer treatment remains in its infancy. In the present study, the anticancer efficacy of a dendrosome-based formulation of polo-like kinase enzyme (PLK-1) specific siRNA was evaluated against DMBA induced skin papillomas in Swiss albino mice. The efficacy of the dendrosome based siRNA nano-formulation was evaluated on the basis of the effect on total numbers and sizes of tumor papillomas and the expression of apoptotic factors. The nano-formulation was successful in reducing the cumulative numbers as well as sizes of tumor nodules and causing significant apoptosis of skin tumor cells which led to better survival of the treated animals. The results indicate that dendrosomes are promising nanocarriers for the development of topical gene silencing approaches to treat skin tumors.
Introduction
Polo-like kinases have been found to be critical regulators of many cell-cycle-related events, including activation of Cdc2, chromosome segregation, centrosome maturation, bipolar spindle formation, regulation of anaphase-promoting complex, and execution of cytokinesis.1–3 Depletion of polo-like kinase 1 (PLK-1), a member of the polo-like kinase family harbouring conserved serine/thronine kinase, has been found to result in mitotic arrest and the inhibition of proliferation, apoptosis, and tumor growth.4 A close correlation between PLK-1 expression and carcinogenesis has now become evident.3 PLK-1 has been found to be over-expressed in various kinds of cancers, including skin cancer.4 Therefore, inhibition of PLK-1 has begun to be realized to have important applications in cancer therapy.Selective silencing of gene expression using small interfering RNA (siRNA) has been investigated as a potential therapeutic approach to cancer. However, as a result of its large molecular weight (MW 13 kDa) and polyanionic nature (40 phosphate groups), naked siRNA does not freely cross the cell membrane, and if however it somehow crosses the membrane, rapid enzymatic degradation, and substantial liver and renal clearance restrict the therapeutic applications of siRNA in vivo.5–8 Consequently, development of efficient delivery systems that protect siRNA from degradation and facilitate uptake by target cells is one of the major challenges in the therapeutic applications of RNAi. Several delivery vehicles including natural as well as synthetic systems have been developed for safe and effective delivery of siRNA. The synthetic carriers basically deliver the synthesized siRNAs (siRNA constructs) to targeted cells, while the bacterial and viral delivery systems are genetically engineered to express shRNAs that in turn undergo intracellular processing to become functional siRNAs. Amongst various natural delivery vehicles, viral-based vectors are highly efficient delivery systems for siRNA; however, the potential for mutagenicity, limited loading capacity, high production costs and, most importantly, safety risks caused by their inflammatory and immunogenic effects severely limit the applicability of virosomes.7 These concerns have led to the development of non-viral synthetic alternatives. Several types of synthetic vectors have been investigated for gene silencing applications, including the development of cationic lipids, liposomes, or both; cationic polymers; cationic dendrimers (branch-like polymer structures); and cationic cell-penetrating peptides.9,10
Dendrosomes, dendrimer encapsulating lipophilic vesicles loaded with either oligonucleotides or conventional drugs have recently begun to be exploited for various therapeutic strategies against cancer. Several groups have demonstrated dendrosomes to exhibit negligible hemolytic toxicity, higher transfection efficiency, and better tolerance in vivo than dendrimers which are highly branched and reactive three dimensional polymers.11–13 Dutta et al. found in one of their pioneering studies that upon complexation of DNA with PAMAM dendrimers, its transfection increased which further improved when dendrimers were loaded into liposomes.11,14 They also reported negligible toxicity of dendrosomes. Reckoning with these attributes of dendrosomes, Dutta et al. further studied dendrosome mediated genetic immunization against hepatitis B. Plasmid DNA encoding pRc/CMV-HBs[S], a small region of the hepatitis B surface antigen, was made to form a complex with dendrimer and further the dendrimer–plasmid DNA complex was entrapped in dendrosomes.15 The results of the study established the potential of dendrosomes for genetic immunization against hepatitis B. Movassaghian et al.13 also established the low-toxicity and higher transfection efficiency of dendrosomes in HeLa cells. In addition to application of dendrosomes as gene vector, it has also been exploited as carrier of drugs which are insoluble in aqueous systems and hence have poor bioavailability in vivo. An example of such a drug is curcumin and dendrosomes have recently been widely explored for its delivery. Mirgani et al.16 recently found that dendrosomal formulation of curcumin successfully downregulates pluripotency genes in glioblastoma cells. Dendrosomal curcumin has also been found to have a chemoprotective effect on breast cancer metastasis through suppression of NF-κB.17 Babaei et al.18 report that treatment of fibrosarcoma bearing mice with dendrosomal curcumin results in significant reduction in tumor size and longer survival than untreated animals.
Considering the very promising results of dendrosomes as gene vectors and drug carriers, researchers have extended their studies to dendrosomal formulations of antisense oligodeoxynucleotides/siRNA. Dendrosomes were recently investigated as carriers of short single strand anti-sense oligodeoxynucleotides targeting PKC-α in non-small cell lung cancer and capable of downregulating the expression of target protein.19 The results of the study highlighted that stable, highly reproducible, oligonucleotide encapsulating dendrosomes can promote efficient cellular uptake of oligonucleotide with no non-specific toxicity. Furthermore, recently, dendrosomes were exploited for the delivery of siRNA.11 siRNA targeting E6–E7 proteins of HeLa cells was used for silencing. The researchers investigated if siRNA loaded dendrosomes would be capable of silencing the E6–E7 genes of HPV in HeLa cells and hence suitable for the treatment of cervical cancer. They found dendrosomes to be non-toxic and capable of delivering siRNA to cervical cancer cells efficiently. Therefore, dendrosomes can be said to hold potential in delivering anti-sense oligonucleotides/siRNA for treatment of cancer.
Topical application is the most attractive approach while dealing with skin tumors because it renders a localized effect at the tumor site and minimizes off-target effects.20 However, topical application of naked siRNA in both normal as well as tumor afflicted tissues is marred by several challenges like lesser absorption into skin owing to cellular and tissue barriers, feeble retention and enzymatic degradation of naked siRNA due to the presence of nucleases in skin.21 Recently, a few reports have shown success in topical delivery of siRNA. SPACE (skin penetrating and cell entering) peptide when conjugated to cargoes such as small molecules and proteins, was able to facilitate their penetration into epidermis and dermis.22 In another study, it has been shown that highly oriented oligonucleotides covalently attached to the surface of gold nanoparticles can readily penetrate epidermal tissues.23
Keeping into consideration the above mentioned details, in the present study, dendrosome mediated topical delivery of siRNA was evaluated in DMBA (7,12-dimethylbenz[a]anthracene) induced papillomas in model animals. The study was performed on the premise that the leaky vasculature of skin papilloma owing to enhanced permeation and retention effect would capture the nanosized siRNA bearing dendrosomes upon their topical administration onto the tumor nodules and may bear potential to suppress skin cancer. The in-house developed dendrosomes were characterized for their size and entrapment efficiency of encapsulated siRNA. Besides survival of cancer inflicted animals, the regulation of anti-/pro-apoptotic protein expression in cancer cells was used as a parameter to establish the efficacy of PLK-1 specific siRNA-bearing dendrosomes.
Materials and methods
Materials
All the reagents used in the study were of highest purity available. siRNA, was purchased from Santa Cruz Company (USA). Anti-p53 wild type (wt), antip53 mut, anti-bax and anti-β-actin antibodies were purchased from BD Biosciences (San Diego, CA). PAMAM dendrimer generation 4 was purchased from Sigma Aldrich (St. Louis, USA). Egg phosphatidylcholine (PC) was purchased from Avanti Polar Lipids (Alabaster, Alabama USA). Cholestrol (C) was purchased from Sigma Aldrich. siRNAs, PLK-1 siRNA (sense strand, 5′-UGAAGAAGAUCACCCUCCUUAdTdT-3′ and antisense strand, 5′-UAAGGAGGGUGAUCUUCUUCAdTdT-3′) and scrambled siRNA (sense strand, 5′-UUCUCCGAACGUGUCACGUdTdT-3′ and antisense strand, 5′-ACGUGACACGUUCGGAGAAdTdT-3′) were supplied by Santa Cruz Biotechnology, Inc., Santa Cruz, CA. All others reagents were of analytical grade and procured from local suppliers.Preparation of dendrimer–siRNA complex
The dendrimer–siRNA complex was prepared by following the protocol as published elsewhere.11 Briefly, 50 nM of siRNA was added to fourth-generation PAMAM dendrimers (N/P ratio 100) in phosphate buffer saline (PBS, pH 7.4) and incubated at 25 °C for 20 min to allow complex formation.Preparation of dendrosome
The in-house prepared dendrimer–siRNA complex was used for the preparation of dendrosomes which were synthesized as described elsewhere.11 Briefly, PC and cholesterol were dissolved in diethyl ether, in varying molar ratios, to which was added the solution of dendrimer–siRNA complex in distilled water. The mixture was sonicated for 5 min at 25 °C, followed by vortexing the thick emulsion to remove traces of organic solvent. The dendrosome so formed was size-reduced by passing through Extruder (Avanti Polar Lipids Inc., Alabaster, Alabama) multiple times (10 times). Purification of dendrosomes was carried out by centrifugation.Characterization of dendrosomes
The dendrosome size and zeta potential were determined by using a Zeta Nano ZN (Malvern Instruments, UK) dynamic light scattering (DLS) detector. The formulation was lyophilized in a 2.0 mL microfuge tube, and the samples were reconstituted in 20 mM phosphate buffer, pH 7.4. The dendrosome suspension was analysed by DLS. The data are the average of three runs of the same sample with standard deviation.Dendrosomes were further characterized by TEM to study their morphological features. TEM samples were prepared following the protocol as published elsewhere24 by placing a drop of synthesized nanoparticles over gold coated negative grid followed by evaporation of the solvent. TEM analysis was performed on JEOL model (1200 EX, JOEL Inc, Peabody, MA) which was operated at an accelerating voltage of 200 kV.
Entrapment efficiency of dendrimer–siRNA complex in dendrosomes
The entrapment efficiency of siRNA in dendrosomes (ratio of the amount of dendrimer–siRNA complex entrapped to the amount of complex added expressed as a percentage) was determined by lysing them with n-propanol. An equal volume of n-propanol was added to a solution of dendrosomes in distilled water and vortexed for 10 min. The resulting solution was centrifuged for 15 min at 2500g. Supernatant was collected and analyzed using UV spectrophotometry at 260 nm after appropriate dilutions against a dendrimer solution as reagent blank.In vitro release kinetics of siRNA from dendrosomes
In order to assess the release kinetics of siRNA from dendrosomes, dendrimer–siRNA complex bearing dendrosomes were dispensed in microvials containing buffer (PBS) of pH 7.4 and pH 6.8. To 1.5 mL vial, 50 μl of dendrosome–siRNA stock formulation (in sterile normal saline) was added and volume was made up to 1 mL with the requisite buffer. The mixture was incubated at 37 °C. Aliquots (100 μl) of supernatant were removed after centrifugation at 9168g for 10 min at different time intervals and were analyzed for siRNA content. Release runs were continued for 7 days. The aliquots were subjected to HPLC analysis to determine siRNA content as described elsewhere.25 The calculated amount of the released siRNA (in%) in PBS (pH 7.4 as well as pH 6.8) was plotted against time.Cytotoxicity studies
Epidermoid cell line procured from NCCS PUNE (India) was maintained in RPMI 1640 media supplemented with 10% FCS, antimycotic and antibiotic solution (sigma) at 37 °C in 5% CO2 atmosphere. Cytotoxicity of dendrimer and dendrosome were assessed on epidermoid cell line following the protocol of MTT assay as published elsewhere.24 Briefly, cells were plated at a density of 2.5 × 104 cells per well in 96 well plate before 24 h of transfection. Cells were incubated with 50 μl of dendrimer and 50 μl of dendrosome in 100 μl of complete medium for 4 h at 37 °C (i.e. 150 μl per well was added). After 4 h of incubation, medium was removed followed by washing with sterile PBS and fresh medium of 100 μl was added. After 24 h of incubation, cell viability was measured by adding 20 μl of MTT dye (5 mg mL−1) in sterile PBS. Plate was incubated further for 3 h at 37 °C. Formazan crystal formed due to reduction was dissolved in DMSO and absorbance was read at 570 nm. The absorption values were expressed as cell viability (%), considering untreated cells as 100%.Analysis of PLK-1 mRNA expression
Cellular levels of PLK-1 mRNA were assessed in epidermoid cell line treated with various siRNA formulations using quantitative real-time PCR (qRT-PCR).26 In qRT-PCR analysis, total RNA from cancer cells was isolated using the RNeasy mini-kits (Qiagen, Germantown, MD) following the manufacturer's instructions. Total RNA (2 μg) was transcribed into cDNA using the cDNA synthesis kit (PrimeScript First Strand cDNA Synthesis Kit, Takara, Japan). Subsequently, qRT-PCR analysis was performed using 2 μl of cDNA targeting PLK-1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) employing the SYBR Premix Ex Taq (Perfect Real Time) (Takara). Analysis was performed using the Applied Biosystems StepOne Real-Time PCR Systems. Relative gene expression values were determined by the ΔΔCT method using StepOne Software v2.1 (Applied Biosystems). Data are presented as the fold difference in PLK-1 expression normalized to the housekeeping gene GAPDH as the internal control, and relative to the cells isolated from untreated control. Primers used are:PLK-1-f: 5′-AGCCTGAGGCCCGATACTACCTAC-3′,
PLK-1-r: 5′-ATTAGGAGTCCCACACAGGGTCTTC-3′,
GAPDH-f: 5′-TTCACCACCATGGAGAAGGC-3′,
GAPDH-r: 5′-GGCATGGACTGTGGTCATGA-3′.
PCR parameters consisted of 30 s of Taq activation at 95 °C, followed by 40 cycles of PCR at 95 °C × 5 s, 60 °C × 30 s, and 1 cycle of 95 °C × 15 s, 60 °C × 60 s, and 95 °C × 15 s. Standard curves were generated and the relative amount of target gene mRNA was normalized to GAPDH mRNA. Specificity was verified by melt curve analysis.
In vitro study of PLK-1 depletion and analysis for the presence of apoptotic factors in epidermoid cell line
Epidermoid cells were incubated with different formulations of siRNA after 80% confluency. After 6 h of incubation with various siRNA formulations, fresh medium containing 20% FCS was added and cells were again incubated for 24 h at 37 °C in 5% CO2 atmosphere. After incubation, the cells were scraped, centrifuged, and washed with RPMI medium and lysed with TNN lysis buffer. The lysate was subjected to 12% SDS PAGE, and gels were electroblotted onto PVDF membrane. The membrane was probed for the presence of p53 wt, p53 mut, bax and PLK-1 using specific antibodies and the bands were developed onto X-ray film by ECL using ECL kit (BioRad).27Animals
Female Swiss albino mice of weight 23 ± 3 g were obtained from the institute's animal house facility. The animals were housed in poly propylene cages on wood powder bedding in an air-conditioned ambience. Animals were quarantined on equal light/dark cycles (12/12 hour) and were kept on a pellet diet (Ashirwad, Chandigarh, India) and water ad libitum. Animals were examined for their mortality and morbidity prior to commencement of the study, and only healthy animals were included in the experiments. The animals which exhibited signs of illness like lethargy, staggered gait, ruffled coat, hunched posture and reduced food intake were humanely euthanized by injecting ketamine/xylazine combination followed by cervical dislocation. Out of total 80 animals procured for the study, 4 animals met the criteria for euthanasia and were euthanized. The healthy animals included were monitored every day at the commencement of the study, however, during the survival study the condition of animals was examined twice a day. Ethics statement. All animal experiments were performed in strict accordance with the National Regulatory Guidelines issued by the Committee for the Purpose of Control and Supervision of Experiments on Animals, Govt. of India (CPCSEA). Our approval ID was 332/CPCSEA, Ministry of Environment and Forest, Government of India. All the procedures used for the animal experiments were reviewed and approved by the Institutional Animal Ethics Committee of the Interdisciplinary Biotechnology Unit, Aligarh Muslim University, Aligarh, India. Animals were anesthetized with ketamine (100 mg kg−1 body weight) in combination with xylazine (5 mg kg−1 body weight) prior to sacrifice by cervical dislocation. In all experimental procedures, efforts were made to minimize pain and suffering.Tumor induction
Skin papilloma was induced in the experimental animals following the published procedure.24 Animals in the resting phase of hair cycle were selected and their hairs were removed from the interscapular region (over an area of 2 cm2) using un-lubricated electric clippers. The skin of the shaved dorsal portion of mice was exposed to DMBA (52 μg in 200 μl acetone) that was applied topically three times a week for 12 weeks. Mice were examined every day for gross morphological changes and development of papilloma. Upon induction of tumor papilloma, the mice were treated with various siRNA formulations.Treatment schedule
For assessment of anticancer efficacy of siRNA bearing dendrosomes, animals were divided into seven different groups each consisting of 15 animals. Group 1 consisted of healthy Swiss albino mice that were not exposed to DMBA and served as positive control. Tumor bearing animals were pooled and divided into six groups. Group 2 consisted of tumor bearing animals without treatment. Group 3 consisted of animals treated with sham (empty) dendrosomes i.e. dendrosomes which did not carry siRNA. Animals belonging to Group 4 were treated with free siRNA. The tumor bearing animals belonging to Group 5 were treated with siRNA complexed dendrimer. Tumor bearing animals of Group 6 were treated with siRNA bearing dendrosome. Animals in the Group 7 were treated with scrambled siRNA loaded dendrosomes that served as negative control.Topical delivery of siRNA
Various siRNA formulations (siRNA–dendrimer, siRNA–dendrosome or free siRNA form) were applied onto the DMBA induced skin papillomas in Swiss albino mice according to the protocol approved by the Institutional Animal Ethics Committee. After induction of papilloma, 200 μl of 50 nM of siRNA in different systems was topically applied over 2 cm2 areas on the back of animal for 10 consecutive days. After application of last dose of various siRNA formulations, animals were analyzed for tumor volume, tumor number, expression of apoptotic factors, histopathology of papillomas, changes in body weight and animal survival at various time points till 12 weeks post treatment.Tumor measurement
The diameter of the tumors was measured using a Digital Vernier Caliper and the tumor size was determined by the formula:| V = D × d2 × π/6 |
All animals were sacrificed and underwent complete skin examination after treatment. The tumor mass was isolated aseptically.
Preparation of nuclear fraction
The skin tumor tissues were removed from experimental mice with sharp scalpel blades.24 The tissue samples were placed on ice and fat was scraped off before further processing. Finally, the samples were homogenized to prepare nuclear fraction.Western blot analysis
Skin papilloma homogenate (30 μg protein) was resolved on 10% SDS-PAGE gel and electroblotted onto PVDF membrane.24 Employing an enhanced chemiluminescence kit, the membrane was probed for the presence of p53 wt, p53 mut, bax and PLK-1 using specific antibodies. Blots were re-probed with an antibody for β-actin and used as a control for equal protein loading and transfer.Determination of caspase-9 level by confocal microscopy
Single cell suspension of skin papilloma cells was permeabilized with 0.1% Triton X-100 in PBS at RT for 10 min followed by fixation in 4% paraformaldehyde for 2 h at room temperature. The fixed cells were blocked with 2% FCS followed by incubation with monoclonal antibody which detects only 35 kDa cleaved caspase 9. Finally, the cells were incubated with FITC tagged 2° antibody. Fluorescence microscopy was performed using Ziess fluorescence microscope at 100× magnification.TUNEL assay
Single cell suspension of skin papilloma cells was prepared by pelleting cells at 300 g for 10 min at 4 °C from various experimental groups. Cells were re-suspended in 1% (w/v) paraformaldehyde in PBS (pH 7.4). Suspension was placed on ice for 30 min followed by centrifugation for 5 min at 300g and supernatant was discarded. The cell pellet was resuspended in 70% (v/v) ice-cold ethanol followed by washing. After keeping for 30 min in 70% ethanol, cells were washed 3 times and pellet was resuspended in 50 μl of DNA labelling solution (reaction buffer + TdT enzyme and Br-dUTP). Cells were incubated in the DNA labelling solution for 60 min at 37 °C in a temperature controlled water bath. Cell pellet was incubated with the FITC-labelled anti-BrdU antibody staining solution in the dark for 30 min at room temperature. After labelling the tumor cells with FITC-tagged anti-BrdU antibody, PI/RNase (50 μg mL−1) was added to the antibody labelled cells which were analyzed by flowcytometer (GUAVA, Millipore, MA, USA) followed by incubation for 30 min at 37 °C.Histopathological studies
To examine effect of PLK-1 siRNA dendrosome on the tumor treatment, three mice from each group were sacrificed by cervical dislocation and fixed in 10% formaldehyde solution. Tumor tissues were then excised and sections were prepared by using conventional paraffin sections and hematoxylin–eosin staining.Humane endpoints during the survival study
The animals in moribund conditions were given humane endpoints during the survival study. The moribund condition is usually defined as an irreversible condition leading inevitably to death. Symptoms of moribundity were (a) unresponsiveness to manual stimulation; (b) loss of mobility; and/or (c) subdued ability to eat or drink. In these conditions, animals were euthanized by injecting ketamine/xylazine combination followed by cervical dislocation and considered being succumbed to death. The animals in the groups ‘untreated control’ (n = 3), ‘dendrosome sham’ (n = 2) and ‘free siRNA’ (n = 2) met the criteria for euthanasia and were euthanized. Animals in other groups spontaneously died and did not meet the criteria for euthanasia.Statistical analysis
Data were analyzed and two groups were compared with the Student t test, and one way ANOVA (Holm-Sidak method) to assess the differences among various groups, using Sigma-Plot version 10 software. P values <0.05 were considered to be significant.Results
Entrapment efficiency and release kinetics of siRNA from dendrosome–siRNA nano-formulation
Dendrimer–siRNA complex encapsulated dendrosome formulation showed approximately 40.12 ± 2.8% entrapment efficiency of PLK-1 specific siRNA. In vitro release kinetics of dendrosomal siRNA was studied at 37 °C in PBS of pH 7.4 as well as pH 6.8. The release kinetics was performed at pH 7.4 and pH 6.8 in order to simulate physiological and tumor ambiences respectively. Dendrosomes showed a sustained release at both the pH conditions. The siRNA release pattern from dendrosomes at pH 7.4 and pH 6.8 was practically same indicating the potential of dendrosomes to tolerate slight changes in pH. Dendrosomes released approx. 15.7% and 17.8% of siRNA in the initial 24 h at pH 7.4 and pH 6.8 respectively that reached to approx. 40% by day 7 for both the pH conditions. Dendrimers too exhibited practically similar release pattern for siRNA at the two pH conditions. However, dendrimers showed a burst release of siRNA in the initial 24 hours as compared to dendrosomes (P < 0.05) with approx. 30% of the entrapped siRNA leaking out (Fig. 1A). Altogether, dendrosomes showed a sustained release of siRNA and were found to exhibit superior release pattern than dendrimers.Characterization of siRNA bearing dendrosomes
The dendrosome–siRNA formulation was first characterized by DLS for size and zeta potential. DLS studies exhibited the size of dendrosomes to be 223 ± 5.7 nm (Fig. 1B) with zeta potential, +4.8 ± 2.1 mV. Surface (zeta) potential of the nanoparticles is considered to regulate their half-life.28 The morphology of the particles was also determined by TEM. As shown in Fig. 1C, the particles appear to have approx. <250 nm size which is in good agreement with DLS studies. The arrow heads in the TEM micrograph depict the branched dendrimers entrapped within lipid vesicle of dendrosomes. Moreover, the particles exhibit a spherical shape.Cytotoxic effect of siRNA-bearing dendrosomes
Cytotoxicity studies using MTT assay were carried out to ascertain the toxicity of the dendrimer and dendrosomes. As shown in Fig. 1D, dendrimers were found to be extremely toxic to epidermoid cells (dendrimer-sham and dendrimer–siRNA vs. untreated control, P < 0.001). However, the empty dendrosomes as well as those encapsulating siRNA were found to be completely nontoxic, clearly demonstrating the superiority of dendrosomes over the dendrimeric formulation. The data shows that the dendrimers resulted in killing of about 75% of the cell population. On the other hand, dendrosomes were found to be toxic to only approx. 7–8% of the cells.PLK-1 mRNA expression is reduced in cells treated with formulations carrying PLK-1 specific siRNA
To analyze if siRNA delivery by the dendrosome formulation affected the knockdown of the target mRNA, we isolated mRNA from the epidermoid cells treated with various siRNA formulations and examined PLK-1 mRNA expression by quantitative real-time PCR. Cells treated with dendrimer–siRNA and dendrosome–siRNA formulation exhibited reduced PLK-1 mRNA levels as compared to untreated control (P < 0.01) (Fig. 1E), while the empty dendrosome preparation (dendrosome-sham) and dendrosome loaded with scrambled siRNA (dendrosome-scr-siRNA) did not cause this effect and exhibited results practically similar to that of untreated control. All the preparations carrying siRNA showed reduced PLK-1 mRNA expression including free form of siRNA, dendrimer–siRNA and dendrosome–siRNA.Effect of dendrosome encapsulated siRNA on apoptosis
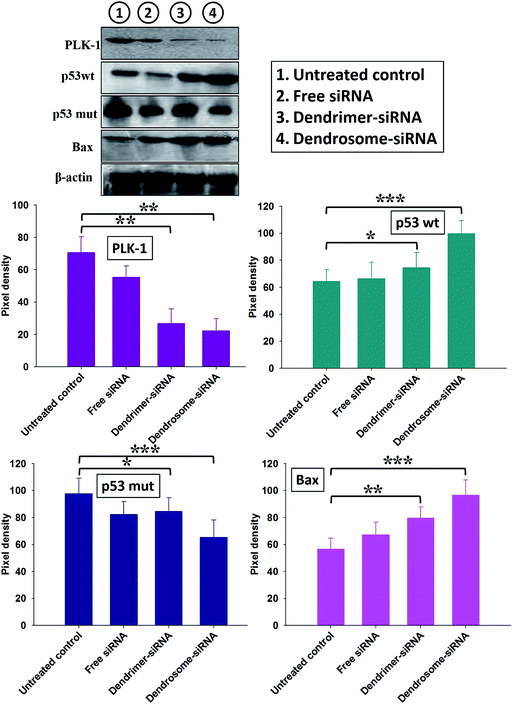
The ability of various siRNA formulations in causing apoptosis of cancer cell line as well as skin tumor cells was evaluated. As shown in Fig. 2, dendrosome–siRNA down-regulated the expression of PLK-1 while enhanced the expression of p53 wild-type (p53wt) and bax in epidermoid cancer cell line. However, other formulations viz. free siRNA and dendrimer–siRNA could also modulate the apoptotic factors in comparison to untreated control but not as effectively as done by dendrosome–siRNA formulation. The results clearly suggest that siRNA while being encapsulated in dendrosomes is delivered efficiently to the cancer cells and can easily modulate various apoptotic factors and eventually result in apoptosis of the cancer cells.Next, we proceeded to analyse the apoptosis of cancer cells in response to administration of various siRNA formulations in mouse model. Results were found to be in concordance with those observed for in vitro studies (Fig. 3). Animals treated with dendrosome–siRNA formulation exhibited marked inhibition of PLK-1 (P < 0.001) in comparison to untreated control. Moreover, the expression of anti-apoptotic molecule; p53 mut and pro-apoptotic molecules; p53 wt and bax was found to be substantially down-regulated and up-regulated respectively in animals treated with dendrosome–siRNA formulation as compared to control groups (P < 0.001).
Effect of dendrosome-encapsulated siRNA on the expression of caspase-9
Skin cancer cells were squeezed out from experimental animals and the efficacy of the siRNA treatment was evaluated by determining the expression of caspase-9 using fluorescence microscopy (Fig. 4). Apoptosis induction was quite visible in free siRNA-treated cells in comparison with dendrosome-sham-treated, dendrosome-scr-siRNA treated and untreated groups, where caspase-9 expression was almost negligible. However, expression of caspase-9 was significantly enhanced in cells isolated from siRNA-bearing dendrosome-treated mice. The group treated with dendrimer–siRNA complex also exhibited a significantly higher expression of caspase-9 than free siRNA but the expression was not as significant as observed for dendrosome–siRNA formulation treated group (Fig. 4).Effect of dendrosome-encapsulated siRNA on DNA fragmentation
Activation of endonucleases during apoptotic events in the cell generally result in DNA fragmentation.17 DNA fragmentation in skin papilloma cells isolated from mice belonging to various experimental groups was observed by TUNEL analysis using FACS (Fig. 5). In a manner similar to fluorescence microscopy results, FACS analysis too revealed that significantly higher apoptosis was induced in cells isolated from dendrosome–siRNA treated group in comparison to free siRNA, dendrimer–siRNA complex, dendrosome-scr-siRNA and untreated control mice (Fig. 5).Histopathological studies
The efficacy of various forms of PLK-1 specific siRNA was established by histopathological studies. Skin tissue samples from mice belonging to various treated groups were used for histopathological analysis. Fig. 6 shows sections of epidermal tissue after treatment with free, dendrimer complexed and dendrosome encapsulated siRNA formulations. The histopathological analysis of tissue treated with various formulations again established higher efficacy of dendrosome encapsulated siRNA. Untreated control animals and animals treated with dendrosome-scr-siRNA demonstrated prominent papillomatous growth having complex fibrovascular core. Heavy keratosis and acanthosis are also observed. The animals treated with free siRNA showed poor response to treatment as both acanthosis and keratosis remain a prominent feature. Treatment with dendrimer–siRNA complex showed moderate response to treatment with reduction in both acanthosis and keratosis. On the other hand, animals treated with siRNA–dendrimer complex entrapped dendrosomes showed the best response to treatment with mild acanthosis and keratosis, and the complexity of fibrovascular core was also almost absent.Effect of dendrosome-encapsulated siRNA formulation on tumor regression, body weight and survival of mice with skin papilloma
The inhouse-prepared dendrosome–siRNA formulation in addition to inducing apoptosis in cancer cells could also regress tumors, normalize the body weight and enhance survival of animals. As shown in Fig. 7, mice treated with dendrosome–siRNA formulation exhibited a significant regression in tumor as compared to control untreated animal at 7th week post treatment. The untreated group shows multiple large pedunculated and papillomatous growth with keratin deposition on the surface in contrast to dendrosome–siRNA treated group that exhibited scantly as well as significantly smaller papillomatous growth. A slight tumor regression was also observed in group administered dendrimer–siRNA complex. However, the groups treated with free siRNA and sham dendrosome exhibited negligible tumor regression. It is also worth noticing that in animals treated with dendrosome–siRNA, a markedly higher tumor regression is observed as compared to other groups with the passage of time post treatment, as can be seen at 1st week and 7th week post treatment (Fig. 7).A quantitative analysis of tumor regression was also performed by counting the average number and volume of tumor upon treatment with various siRNA formulations. As observed in Fig. 8A and B, the average number as well as volume of skin papillomas were significantly reduced in the dendrosome–siRNA treated group as compared to untreated control (P < 0.001). Dendrimer–siRNA complex also exhibited a moderately higher tumor regression in terms of reduction in number and size as compared to control (P < 0.01). The sham dendrosome, dendrosome-scr-siRNA and free siRNA groups were devoid of any significant tumor regression activity as compared to control untreated group. Next, the efficacy of various siRNA formulations was assessed in terms of improvement in body weight and survival of treated animals. The treatment with dendrosomal siRNA prevented weight loss and also mitigated the small reduction to a significant extent (in comparison to control untreated animals) (P < 0.001). Dendrimer–siRNA complex also mitigated the weight loss but not as significantly as observed for dendrosome–siRNA formulation (P < 0.05). The group administered free siRNA lost weight gradually (were unsuccessful in mitigating the weight loss) but the loss was highly reduced as compared to control untreated animals and mice belonging to dendrosome-sham group so that it got reduced to approx. 14 g after 6 weeks of treatment (Fig. 8C). Mice belonging to untreated control and dendrosome-scr-siRNA groups, and dendrosome-sham group succumbed to death after 7 and 8 weeks respectively and were not available for further weight measurements.
Survival graph shows the augmentation of anticancer efficacy of dendrosome–siRNA formulation against DMBA-induced tumorigenesis at different time points. The animals treated with dendrosome–siRNA formulation exhibited 80% survival, whereas the group receiving dendrimer–siRNA complex resulted in 60% survival after 12 weeks of treatment. Free siRNA administered group showed only 20% survival (dendrosome–siRNA vs. free siRNA, P < 0.001; dendrimer–siRNA vs. free siRNA, P < 0.05). None of the animals survived beyond 9 weeks in the control groups viz. sham dendrosome, dendrosome-scr-siRNA administered groups and untreated animals (Fig. 8D).
Discussion
Administering siRNA holds great potential for treatment of various diseases. In fact, selective blocking of the gene of interest using antisense DNA and siRNA has delivered promising results as substantiated from many ongoing clinical trials for a variety of diseases including cancer.23,29 For treatment of skin disorders including cancer by gene suppression, topical delivery of siRNA is the pre-requisite. However, penetration of antisense DNA/siRNA into the epidermal barrier remains a major stumbling block that needs to be circumvented in order to harness the therapeutic potential of oligonucleotides in skin. In order to accomplish the challenges of topical delivery of siRNA, various suitable delivery vehicles have been proposed.23 Recently, topical delivery of siRNA has been accomplished employing gold nanoparticles covalently conjugated to highly oriented siRNA. While dealing with skin cancer, topical administration of therapeutic oligonucleotides in nanodelivery systems bears many advantages.• Easy accessibility of skin and the reduced risk of systemic side effects
• protection from nucleases secreted on the skin
• preventing non-specific delivery and enhancing targeted cargo unloading
• most importantly, avid uptake of the oligonucleotide carrying nanoparticles (<400 nm) by the leaky vessels (a general feature of cancerous tissues) due to enhanced permeation and retention (EPR) effect
• enhanced retention at the target site owing to poor lymphatic drainage (an attribute of tumor).
Various delivery systems have been tested till date for gene delivery and genetic immunization, which in addition to liposomes and other nanoparticles includes dendrimers. Dendrimers are highly branched and reactive three-dimensional nano-polymers. The reactivity of dendrimers is owed to the presence of numerous positively charged amine groups at the periphery of the polymer that can interact with negatively charged oligonucleotides imparting them potential to act as gene delivery agents.15,30 Therefore, in recent years dendrimers have attracted enormous attention among researchers working in the field of gene or siRNA delivery. However, the dendrimers are reported to possess hemolytic toxicity and cytotoxicity owing to their polycationic nature. Moreover, interaction with the oppositely charged macromolecules in plasma results in premature release of nucleic acid within the blood. Also, degradation of the nucleic acid by nucleases present in the plasma leads to poor efficacy of dendrimer–nucleic acid complex in vivo.
Reckoning with the inherent toxicities and reduced efficacy of dendrimers in vivo, scientific community has recently shifted its focus to dendrosomes which are dendrimer encapsulating lipophilic vesicles.31,32 The lipophilic shell of dendrosomes offers protection to siRNA complexed to dendrimers and also circumvents the toxicity of dendrimers since their encagement in vesicles masks the cationic amines to interact with extracellular milieu. Moreover, dendrosomes being lipophilic nanostructures are biodegradable and do not evoke immunological response, therefore, providing added advantages for in vivo delivery. Dendrosome encapsulated siRNA have been recently found to be effective against cancer cell lines.11 Considering the above facts, we evaluated the potential of dendrimer–siRNA complex encapsulated dendrosomes in treating DMBA-induced papilloma in model animals. DMBA, a polycyclic aromatic hydrocarbon, forms a DNA adduct and initiates carcinogenesis.33 We had chosen PLK-1 specific siRNA since earlier studies have demonstrated the potential of PLK-1 specific siRNA in suppression of tumor growth.3,34–36 We too have recently demonstrated the efficacy of PLK-1 specific siRNA in treating liver cancer in model animals.25,37
The data of the present study establish higher efficacy of dendrosome–siRNA formulation which was assessed on the basis of its ability to reduce total numbers as well as sizes of tumor papillomas formed, modulate pro/anti-apoptotic factors and enhance survival of the treated animals. A schematic representation showing efficacy of dendrosomal siRNA in the treatment of skin papilloma is shown in Fig. 9. The dendrosomes showed a sustained release pattern of siRNA in physiological as well as acidic ambiences (Fig. 1A). The two pH conditions were selected in order to analyse the fate of dendrosome–siRNA in physiological as well as tumoral milieu. The siRNA release pattern of dendrosomes was found to be superior to that exhibited by dendrimers possibly owing to the dual-delivery system presented by dendrosomes wherein dendrimer entrapped siRNA are further encapsulated within lipid vesicles. Overall, the dendrosomes acted as a sustained release system allowing greater accumulation of siRNA molecules at the tumor site. Although the leaky vasculature of the cancerous tissues favours uptake of particulate material but the capture is highly dependent upon the size of the particles. The leaky vessels of cancerous tissues allow particles of approx. 400 nm size to excavate.38 Moreover, the size of particles is also important when phagocytosis is taken into account. Therefore, we analysed the size of dendrosome–siRNA formulation. DLS and TEM revealed the mean size of the dendrosome–siRNA formulation to be approx. 225 nm (Fig. 1B and C) indicating that they can easily excavate leaky vessels in tumor, which could be another reason for increased efficacy of dendrosome-siRNA formulation. The zeta potential of nanoparticles plays an important role in regulating their half life, therefore, we also determined the zeta potential of dendrosome–siRNA formulation which was found to be +4.8 ± 2.1 mV. Cytotoxic studies overruled the toxicity of dendrosomes (Fig. 1D). Therefore, the higher efficacy of dendrosome–siRNA formulation can be solely attributed to the effective delivery of siRNA in the tumor vasculature by dendrosomes. On the other hand, dendrimers exhibited a marked toxicity which indicates that the anticancer potential observed for dendrimer–siRNA formulation can be in part because of the dendrimers instead of effective siRNA mediated knockdown. The non-specific toxicity of dendrimers poses a risk to normal healthy tissues.
 | ||
| Fig. 9 Schematic illustration showing potential of dendrosome–siRNA in treatment of skin papilloma in mouse models. | ||
Further, before proceeding to in vivo studies, we verified whether the dendrosome–siRNA formulation is capable of efficiently knocking down the expression of the target therapeutic gene PLK-1 in epidermoid cell line. We found that the formulations carrying PLK-1 siRNA including dendrosome–siRNA formulation effectively knocked down the expression of the target gene, however, negative controls such as dendrosome-sham and dendrosome-scr-siRNA showed no knockdown efficiency (Fig. 1E). PLK-1 mRNA knockdown was subsequently paralleled by reduced PLK-1 protein expression in epidermoid cell line as analyzed by Western blot (Fig. 2). Concordantly, PLK-1 expression was found to be significantly down-regulated in skin cancer cells isolated from animals treated with dendrosome–siRNA (Fig. 3).
Further, the study was extended to gain insight in dendrosome–siRNA mediated modulation of various proteins involved in the apoptosis of cancer cell line as well as tumor cells of various experimental groups. The dendrosome–siRNA formulation was successful in downregulating the expression of target protein PLK-1 and therefore enhancing the expression of p53wt and bax in cell line as well as skin cancer cells (Fig. 2 and 3). On the other hand, free siRNA or dendrimer–siRNA complex could feebly modulate the expression of these proteins. Silencing of PLK-1 has been demonstrated to reinstate normal production of various pro-apoptotic molecules (p53 and bax) and down-regulate expression of anti-apoptotic molecules (p53 mut). In cancer cell line as well as tumor cells isolated from various experimental groups, comparatively higher expression of pro-apoptotic proteins was observed in the cells treated with siRNA-bearing dendrosomes compared with free siRNA and dendrimer–siRNA administered groups. The data clearly suggest that siRNA-bearing dendrosomes play a determining role in upregulation of apoptotic molecules and aborting the up-regulation of anti-apoptotic molecules. Also, expression of caspase-9 was found to be enhanced upon treatment with dendrosome–siRNA nanoparticles compared with the free siRNA formulation (Fig. 4). Caspase 9 is responsible for activating effector caspases, caspase-3 and caspase-7, which further activate the downstream signaling cascade and induce apoptosis. An augmented apoptosis induction in the cancer cells by dendrosome–siRNA formulation in comparison to free siRNA and dendrimer–siRNA complex was further supported by APO-BrdU™ TUNEL assay employing FACS analysis (Fig. 5).
Moreover, the histopathological analysis of tissue from various treated groups of mice also established superior efficacy of dendrosome–siRNA nanoparticles over other siRNA formulations. Histopathological studies of skin revealed prominent recovery and preservation of general architecture of epidermal tissue upon treatment with dendrosome–siRNA formulations (Fig. 6). Administration of dendrosome–siRNA formulation resulted in an efficient suppression of tumor growth, significant mitigation of the weight loss of cancer inflicted animals and enhanced survival in comparison to the naked dendrimer–siRNA complex (Fig. 7 and 8). This clearly suggests that putting the dendrimer–siRNA complex in a lipophilic shell offers an effective strategy to augment the protective efficacy of PLK-1 specific siRNA against skin papillomas. Knockdown of PLK-1 gene by siRNA has been reported to down-regulate MDR1 expression and induce apoptosis of cancer cells.39 PLK-1 siRNA loaded dendrosomes may have accumulated at the tumor site owing to the leaky vasculature of the tumor and would have delivered PLK-1 siRNA there over time in a slow and sustained manner. This may have resulted in enhanced apoptosis of cancer cells in dendrosome–siRNA treated animals inhibiting disease progression and leading to augmented tumor regression and enhanced survival.
It is a well-known fact that efficient cellular uptake of cationic polymers like dendrimers relies on a slight excess of positive charge, which allows binding of the dendrimer–nucleic acid complexes with the anionic cell membrane and its subsequent internalization. However, the cationic charge on the surface of the complex can be masked by the skin tissue proteins while delivering it topically, which impose a net negative charge on the surface of the delivery system. Moreover, the siRNA in dendrimer–siRNA complex is less protected than in dendrosomes because of the extra shield provided by the lipophilic shell in dendrosomes. Observations that anionic molecules can act competitively to release siRNA from complexes with cationic vesicles or molecules raises the interesting possibility that in the skin negatively charged components (e.g. proteins) may interfere by competing with siRNA for binding sites, thus bringing about its premature release. For the dendrimer–siRNA complex entrapped within the dendrosomal vesicles, the anionic molecules not only fail to compete for binding sites but the siRNA is also protected from degradation by nucleases present in the epidermal tissue. This can be the reason behind overall observed greater efficiency of dendrosomal siRNA formulation as compared to that of free siRNA and dendrimer–siRNA complex.
Therefore, it can be concluded that in contrast to naked siRNA or siRNA–dendrimer complex administration, topical administration of dendrosomal formulation of PLK-1 specific siRNA has a different fate. There is considerable degradation of naked siRNA by skin nucleases with only a fraction of surviving materials reaching the target site and therefore a large dose is required to attain matching protective efficacy as observed for dendrosomal siRNA formulation. In dendrosomes, the vesicular layers largely protect the dendrimer–siRNA complex. Our observations clearly prove the superiority of dendrosomes over dendrimer–siRNA complex and free siRNA for topical siRNA delivery to combat skin papilloma.
Considering the results of this study, dendrosomal siRNA formulation can also be tested against various other types of cancers. The nanosized dendrosomes can be uptaken by leaky vessels by EPR effect, leading to passive targeting of the loaded cargo as in the present case. The outcome can further be improved by active targeting of dendrosomes to the cancerous site employing target specific antibodies or ligands. The outer layer of dendrosomes being composed of liposomes can be functionalized with desired ligands.40 However, following considerations/obstructions are to be taken into account. The target antigen if not expressed adequately can hinder with the targeting, therefore, the target antigen needs to be sufficiently expressed on the tumor cells to facilitate substantial binding of dendrosomes with cancer cells. Modification of ligands tagged onto dendrosome surface may also pose a problem, hence, unhindered interaction of dendrosomes may be facilitated by avoiding over-modification of attached ligands. Dendrosomes can be PEGylated similar to liposomes and decorated with antibodies, however, the quantity of attached antibodies need to be checked to avoid compromising longevity. Further, internalizable ligands may facilitate intracellular delivery of siRNA which may lead to improved therapeutic outcome.
Conflict of interest
Authors report that no conflict of interest exists.Author contributions
Conceived and designed the experiments: MAS ST. Performed the experiments: MAS ST AAK. Analyzed the data: MAS ST MO. Wrote the paper: MAS ST.Acknowledgements
The authors would like to gratefully acknowledge former and present Co-ordinators, Interdisciplinary Biotechnology Unit and University Sophisticated Instrumentation Facility, AMU for providing with the facilities to accomplish the study. MAS and ST gratefully acknowledge Department of Biotechnology (DBT) and Council of Scientific and Industrial Research (CSIR) respectively for granting Senior Research Fellowship (SRF).References
- D. M. Glover, M. Hagan and A. A. M. Tavares, Genes Dev., 1998, 12, 3777–3787 CrossRef CAS PubMed.
- E. A. Nigg, Curr. Opin. Cell Biol., 1998, 10, 776–783 CrossRef CAS PubMed.
- X. Liu and R. L. Erikson, Proc. Natl. Acad. Sci. U. S. A., 2003, 100(10), 5789–5794 CrossRef CAS PubMed.
- Y. Degenhardt and T. Lampkin, Clin. Cancer Res., 2010, 16(2), 384–389 CrossRef CAS PubMed.
- K. A. Howard, U. L. Rahbek, X. Liu, C. K. Damgaard, S. Z. Glud, M. Ø. Andersen, M. B. Hovgaard, A. Schmitz, J. R. Nyengaard, F. Besenbacher and J. Kjems, Mol. Ther., 2006, 14(4), 476–484 CrossRef CAS PubMed.
- P. S. Kowalski, N. G. Leus, G. L. Scherphof, M. H. Ruiters, J. A. Kamps and G. Molema, IUBMB Life, 2011, 63(8), 648–658 CrossRef CAS PubMed.
- K. F. Badrealam, S. Zubair and M. Owais, Curr. Gene Ther., 2015, 15, 201–214 CrossRef CAS PubMed.
- G. Navarro, J. Pan and V. P. Torchilin, Mol. Pharm., 2015, 12(2), 301–313 CrossRef CAS PubMed.
- Y. Gao, X. L. Liu and X. R. Li, Int. J. Nanomed., 2011, 6, 1017–1025 CrossRef CAS PubMed.
- O. Taratula, R. Savla, H. He and T. Minko, Int. J. Nanotechnol., 2011, 8, 36–52 CrossRef CAS.
- T. Dutta, M. Burgess, N. A. McMillan and H. S. Parekh, Nanomedicine, 2010, 6(3), 463–470 CrossRef CAS PubMed.
- T. Dutta, H. B. Aghase, P. Vijayarajkumar, M. Joshi and N. K. Jain, J. Exp. Nanosci., 2006, 1(2), 235–248 CrossRef CAS.
- S. Movassaghian, H. R. Moghimi, F. H. Shirazi and V. P. Torchilin, J. Drug Targeting, 2011, 19(10), 925–932 CrossRef CAS PubMed.
- C. M. Paleos, D. Tsiourvas, Z. Sideratou and A. Pantos, J. Controlled Release, 2013, 170, 141–152 CrossRef CAS PubMed.
- T. Dutta, M. Garg and N. K. Jain, Vaccine, 2008, 26, 3389–3394 CrossRef CAS PubMed.
- M. T. Mirgani, B. Isacchi, M. Sadeghizadeh, F. Marra, A. R. Bilia, S. J. Mowla, F. Najafi and E. Babaei, Int. J. Nanomed., 2014, 9, 403–417 Search PubMed.
- B. Farhangi, A. M. Alizadeh, H. Khodayari, S. Khodayari, M. J. Dehghan, V. Khori, A. Heidarzadeh, M. Khaniki, M. Sadeghiezadeh and F. Najafi, Eur. J. Pharmacol., 2015, 758, 188–196 CrossRef CAS PubMed.
- E. Babaei, M. Sadeghizadeh, Z. M. Hassan, M. A. Feizi, F. Najafi and S. M. Hashemi, Int. Immunopharmacol., 2012, 12(1), 226–234 CrossRef CAS PubMed.
- S. Movassaghian, H. R. Moghimi, F. H. Shirazi, A. Koshkaryev, M. S. Trivedi and V. P. Torchilin, Int. J. Pharm., 2013, 441(1–2), 82–91 CrossRef CAS PubMed.
- V. V. Venuganti, M. Saraswathy, C. Dwivedi, R. S. Kaushik and O. P. Perumal, Nanoscale, 2015, 7(9), 3903–3914 RSC.
- M. Pan, J. Ni, H. He, S. Gao and X. Duan, BMB Rep., 2015, 48(3), 147–152 CrossRef CAS PubMed.
- T. Hsu and S. Mitragotri, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(38), 15816–15821 CrossRef CAS PubMed.
- D. Zheng, D. A. Giljohann, D. L. Chen, M. D. Massich, X. Q. Wang, H. Iordanov, C. A. Mirkin and A. S. Paller, Proc. Natl. Acad. Sci. U. S. A., 2012, 109(30), 11975–11980 CrossRef CAS PubMed.
- M. Farazuddin, B. Sharma, A. A. Khan, B. Joshi and M. Owais, Int. J. Nanomed., 2012, 7, 35–47 CAS.
- A. Chauhan, S. Zubair, A. Nadeem, S. A. Ansari, M. Y. Ansari and O. Mohammad, Nanomedicine, 2014, 9, 407–420 CrossRef CAS PubMed.
- M. A. Sherwani, S. Tufail, A. A. Khan and M. Owais, PLoS One, 2015, 10(7), e0131684 Search PubMed.
- S. Tufail, M. Owais, S. Kazmi, R. Balyan, J. K. Khalsa, S. M. Faisal, M. A. Sherwani, M. A. Gatoo, M. S. Umar and S. Zubair, J. Biol. Chem., 2015, 290(7), 4131–4148 CrossRef CAS PubMed.
- A. Khan, A. A. Khan, V. Dwivedi, M. G. Ahmad, S. Hakeem and M. Owais, Mol. Med., 2007, 13, 266–276 CAS.
- E. R. Rayburn and R. W. Zhang, Drug Discovery Today, 2008, 13, 513–521 CrossRef CAS PubMed.
- A. K. Patri, J. F. Kukowska-Latallo and J. R. Baker Jr, Adv. Drug Delivery Rev., 2005, 57(15), 2203–2214 CrossRef CAS PubMed; M. N. Sarbolouki, M. Sadeghizadeh, M. M. Yaghoobi, A. Karami and T. Lohrasbi, J. Chem. Technol. Biotechnol., 2000, 75, 919–922 CrossRef.
- M. N. Sarbolouki, M. Sadeghizadeh, M. M. Yaghoobi, A. Karami and T. Lohrasbi, J. Chem. Technol. Biotechnol., 2000, 75, 919–922 CrossRef CAS.
- N. A. Balenga, F. Zahedifard, R. Weiss, M. N. Sarbolouki, J. Thalhamer and S. Rafati, J. Biotechnol., 2006, 124(3), 602–614 CrossRef CAS PubMed.
- V. J. Melendez-Colon, A. Luch, A. Seidel and W. M. Baird, Carcinogenesis, 1999, 20(10), 1885–1891 CrossRef CAS PubMed.
- R. Pellegrino, D. F. Calvisi, S. Ladu, V. Ehemann, T. Staniscia, M. Evert, F. Dombrowski, P. Schirmacher and T. Longerich, Hepatology, 2010, 51(3), 857–868 CAS.
- L. Cheng, C. Wang and J. Jing, Curr. Pharm. Des., 2015, 21(10), 1347–1350 CrossRef CAS PubMed.
- M. Nogawa, T. Yuasa, S. Kimura, M. Tanaka, J. Kuroda, K. Sato, A. Yokota, H. Segawa, Y. Toda, S. Kageyama, T. Yoshiki, Y. Okada and T. Maekawa, J. Clin. Invest., 2005, 115(4), 978–985 CrossRef CAS PubMed.
- M. A. Sherwani, S. Tufail, A. A. Khan and M. Owais, RSC Adv., 2015, 5, 39512–39531 RSC.
- Y. H. Bae and K. Park, J. Controlled Release, 2011, 153(3), 198–205 CrossRef CAS PubMed.
- Y. Tian, Y. Zhang, J. Pan, N. Lu, S. Wang and G. Lu, J. Nanomater., 2015, 2015, 720198 Search PubMed.
- R. R. Sawant and V. P. Torchilin, AAPS J., 2012, 14(2), 303–315 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |