Fabrication of a nutrient delivery system of docosahexaenoic acid nanoemulsions via high energy techniques†
P. Karthikab and
C. Anandharamakrishnan*ab
aFood Engineering Department, CSIR-Central Food Technological Research Institute, Mysore-570 020, India. E-mail: anandhram@cftri.res.in; c.anandharamakrishnan@gmail.com; Fax: +91-821-2517233; Tel: +91-821-2513910
bAcSIR-Academy of Scientific and Innovative Research, CSIR-CFTRI Campus, Mysore-570 020, India
First published on 17th December 2015
Abstract
Docosahexaenoic acid (DHA) is the key omega-3 fatty acid for the growth and development of the human brain and retina and is inevitable for heart health. DHA is highly susceptible to environmental factors due to its increased oxidation and physical instability; therefore, preventing and extending its shelf life is highly required. DHA can be made stable in terms of its chemical and kinetic attributes with the help of nanoemulsion systems. In this study, a DHA nanoemulsion was prepared via an oil-in-water emulsion using Tween-40 emulsifier, through high speed homogenization (HSH), high pressure homogenization (HPH) and combination of the HSH + HPH techniques. The stability of the nanoemulsion was investigated using physiochemical methods under different storage conditions. The fabricated nanoemulsions were less than 88 nm in size with globular droplets having higher negative charges of −31.1 and −30.2 mV, which were produced by the combination and HPH technique, respectively. Higher Newtonian flow behavior was observed for the combination and HPH technique. The functional groups of DHA remained intact without undergoing any changes and functional activity during the nanoemulsification process, which was evident from the unchanged fatty acids profile. Similarly, there were no structural changes observed in all the DHA emulsions. The refrigerated (4 ± 1 °C) emulsion exhibited lower lipid oxidation than those stored at the temperature of 28 ± 1 °C and 40 ± 0.2 °C. Combination of the homogenization techniques was found to result in better physiochemical properties and stability over a period of 100 days during storage than the other emulsification techniques for the encapsulation of DHA.
Introduction
Several studies have confirmed the benefits of omega-3 fatty acids (EPA, C20:5n-3 and DHA) supplementation in case of pregnancy, cardiovascular diseases, and inflammatory disease. Docosahexaenoic acid (DHA; C22:6n-3) plays a vital role in proper cell membrane function and the development of fetal brain and retina during pregnancy.1,2 The human body cannot synthesize essential fatty acids; therefore, it should be supplied from external sources. However, a major problem associated with DHA is oxidation due to its high poly unsaturation. Furthermore, it is not stable for long periods due to environmental conditions such as temperature, moisture, air, pH, and light. To overcome these problems, encapsulation through nanoemulsion is the solution to prevent the effects of environmental factors and increase the stability of DHA.A nanoemulsion is a colloidal system, because one phase is dispersed into another phase in tiny droplets, which can be either an oil or water phase that resides between two immiscible liquids. In the case of the oil-in-water emulsion system, the oil is dispersed into the water phase. Because their free energy of formation is greater than zero, nanoemulsions are kinetically stable systems3 but their long term physical stability gives them unique characteristics and sometimes thermodynamic stability.4 Moreover, they exhibit great potential to encapsulate a high concentration of oil-soluble nutraceuticals or bioactive food compounds for fortification into food systems.5 Due to the inherent characteristics of their tiny droplet size and higher surface area, nanoemulsions can improve the bioavailability and solubility of encapsulated components (carotenoids, polyunsaturated fatty acids, vitamin-E, and polyphenols) for delivery systems.6,7 Therefore, nanoemulsions can be a primary process for the production of dried nanoencapsulated powders through conventional drying methods such as spray drying or freeze drying.8
In food emulsions, two techniques are used to prepare nanoemulsions, which are the low-energy emulsification (phase inversion temperature and phase inversion composition) and high-energy emulsification (high-speed homogenization, high-pressure homogenization, microfluidization and ultrasonication) techniques. In addition, the low-energy methods are the spontaneous formation of very fine oil droplets under the influence of a specific system, whereas the high-energy methods rely on intense mechanical forces to breakdown the macroscopic phases or oil droplets into smaller droplets with help of mechanical devices.9
To produce tiny droplets in an emulsion system, additional shear force is applied using a high speed homogenizer. At the applied shear force, a longer time is required to breakdown the oil droplets. In the case of high-pressure homogenization, the combination of intense shear, cavitation and turbulent flow are involved to create tiny oil droplets.10 Moreover, HPH yields a uniform droplet size distribution and low mean particle diameter in nanoemulsions, which both result in high stability, whereas the opposite holds true in the case of HSH, which results in lower emulsion stability. This is the basis of the combination technique, which utilizes a two step process involving high speed (primary emulsification) and high pressure (secondary emulsification) techniques. During high speed homogenization, oil droplet breakdown can be achieved gradually over a longer period. Subsequently, the high pressure technique reduces the process time and obtains a monomodal droplet size distribution. However, emulsification via the combination of high speed and high pressure could be an effective method for the production of nanoemulsions with a lower droplet size and higher stability.
The objective of this study is to investigate different nanoemulsification techniques to produce stable DHA nanoemulsions using HSH, HPH and combination of the HSH + HPH techniques. Furthermore, the effectiveness of stability is studied in terms of storage stability, morphology, particle size, particle charge, thermal transition, oxidation, fatty acid composition and structural changes.
Materials and methods
Preparation of coarse emulsion
The oil in water nanoemulsion was prepared by the addition of DHA algae oil (10%, w/w; Martek Bioscience, Kingstree, SC) to an aqueous solution containing Tween-40 emulsifier (2.8%, w/w; Sigma Aldrich, Bangalore, India). Before using this phase composition, the emulsifier and oil ratio was varied to formulate a stable emulsion. These two phases were mixed using a high-speed homogenizer (T18 digital Ultra Turrax, IKA, Bangalore, India) at 1000 rpm for 5 min to prepare the coarse emulsion. After the preparation of the coarse emulsion, the nanoemulsions were prepared using different emulsification techniques.Preparation of nanoemulsion by HSH
In the preliminary study, different rpm (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 20
000, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 24
000, and 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and time intervals (15, 20, and 25 min) were used to produce nanoemulsions. After the preparation, the nanoemulsion particle size was measured with respect to different parameters. The obtained results showed that the emulsions prepared at 24
000) and time intervals (15, 20, and 25 min) were used to produce nanoemulsions. After the preparation, the nanoemulsion particle size was measured with respect to different parameters. The obtained results showed that the emulsions prepared at 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and 25 min of homogenization led to a lower mean particle when compared to the other homogenization speed–time combinations. Therefore, based on the optimized results, the above mentioned conditions were used for the HSH operation. The prepared course emulsion was vigorously homogenized at 24
000 rpm and 25 min of homogenization led to a lower mean particle when compared to the other homogenization speed–time combinations. Therefore, based on the optimized results, the above mentioned conditions were used for the HSH operation. The prepared course emulsion was vigorously homogenized at 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 25 min to prepare the nanoemulsion using a high speed homogenizer. This experiment was performed under cold conditions (4 ± 1 °C) to prevent heat generation during homogenization.
000 rpm for 25 min to prepare the nanoemulsion using a high speed homogenizer. This experiment was performed under cold conditions (4 ± 1 °C) to prevent heat generation during homogenization.
Preparation of nanoemulsion via the HPH technique
The high pressure homogenizer system (GEA Niro Soavi, Panda, NS1001L2K, Italy) was optimized before the production of nanoemulsions by varying the pressure (500, 600, 700 and 800 bar) and cycle (5, 6, 7 and 8). However, 800 bar with 8 cycles was chosen based on the lower mean droplet size, poly dispersity index (PDI) and uniform droplet size distribution. With an increase in pressure (e.g. 900, 1000 and 1100 bar) and no. of cycles (e.g. 9, 10 and 11), there are more chances to affect the stability of DHA during the nanoemulsion preparation. This instability is mainly attributed to the higher disruption and physical stress during the homogenization process. In addition, the high pressure and increased number of cycles will increase the oxidation of DHA. After the optimization process, the course emulsion was passed through the high pressure homogenizer at 800 bar for 8 cycles to prepare the nanoemulsion. This experiment was performed under cold conditions (4 ± 1 °C) to prevent heat generation during homogenization.Preparation of nanoemulsion via the combination (HSH + HPH) technique
The prepared course emulsion was homogenized using the combination of high speed homogenization at 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min (primary emulsion) and high pressure homogenization at 800 bar for 8 cycles to form a stable nanoemulsion (secondary emulsion). This experiment was performed under cold conditions (4 ± 1 °C) to prevent heat generation during homogenization. After the preparation, all three types of DHA nanoemulsions were kept in screw cap glass tubes for further analysis.
000 rpm for 15 min (primary emulsion) and high pressure homogenization at 800 bar for 8 cycles to form a stable nanoemulsion (secondary emulsion). This experiment was performed under cold conditions (4 ± 1 °C) to prevent heat generation during homogenization. After the preparation, all three types of DHA nanoemulsions were kept in screw cap glass tubes for further analysis.
Determination of zeta potential and particle size distribution
The electrical charge (ζ-potential) of the nanoemulsion oil droplets was determined using a Malvern Zetasizer (Nano-ZS90; Malvern Instruments, U.K.). The nanoemulsions particle size distribution was measured via the dynamic light scattering technique using the same instrument. Refractive indices of 1.48 for oil and 1.33 for dispersant medium were used to determine the particle size of the emulsions. The analysis of particle size distribution from the emulsion stored on the 100th day was carried out using a laser light diffraction particle size analyzer (S3500, Microtrac Inc., USA). This experiment was performed in triplicate.Morphological studies of nanoemulsions
Rheological characteristics
Rheological characterization of DHA algae oil nanoemulsions were performed using a rheometer (Rheomatric Scientific, SR5, USA) with a coaxial cylinder (28.8 mm size diameter and 50 mm length) attachment. The shear rate was gradually increased from 0.1 to 100 s−1 in a span of 60 s. A pre-shearing process was done for 30 s at 10 s−1 and the waiting time process was maintained during every test for 40 s. The temperature was maintained at 25.0 ± 0.1 °C for all the experiments and the rheological measurements were conducted in triplicate.Storage stability studies at different temperatures
Storage stability studies on the DHA nanoemulsions (HSH, HPH and HSH + HPH) were performed at different storage conditions such as refrigeration (4 ± 1 °C), room (28 ± 1 °C) and oven temperature (40 ± 0.2 °C). This study was carried out to determine the effect of temperature on the nanoemulsions stability during storage. The nanoemulsions changes were observed via morphology and different physiochemical studies.Creaming stability
The nanoemulsions long term creaming stability was studied via visual observation. The prepared nanoemulsions (20 mL) were stored in a transparent measuring cylinder with a stopper. Their creaming stability was determined in samples stored for 1, 3, 5, 8, 10, 20, and 100 days. It was observed that the emulsions separated into an opaque layer at the top (cream) and a marginally turbid or transparent layer at the bottom (serum). Emulsion creaming was monitored by measuring the height of the cream layer on top (HC) and the height of total emulsion (HE) in the tube. Creaming stability in terms of creaming index (%) was obtained using the following eqn (1):11
 | (1) |
Centrifugation, phase separation and sedimentation stability
Phase separation experiments under centrifugation conditions (2 mL of emulsion, 28 ± 1 °C at 3000 rpm for 2 min; Eppendorf, 5430 R, Germany) were carried out for all the prepared DHA nanoemulsions.12 Similarly, phase separation under normal conditions without centrifugation and sedimentation was studied for all the emulsions. These measurement studies were performed at different storage time intervals such as 1, 2, 3, 5, 10, 40 and 100 days.Flocculation, coalescence and oiling off stability
Nanoemulsion samples were transferred into 20 mL glass test tubes and placed in different storage conditions over the storage period of 100 days. Flocculation and coalescence were studied by the observation of emulsion structure using a trinocular microscope. All the emulsion samples were observed under 100× magnification with oil immersion. The oiling off of the emulsion sample was determined by measuring the height of the oil layer in the emulsion samples (H0) and total height of the oil layer in a completely destabilized system (Htotal). Oiling off was obtained using the following eqn (2):13
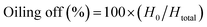 | (2) |
Color analysis
The color (CIE L* a* b*; where, L*-lightness, a*-redness, and b*-yellowness) of the DHA nanoemulsions was evaluated using a color measurement analyzer (MINOLTA Spectrophotometer CM-3500d, Spectra Magic software). The nanoemulsions were analyzed at the different time intervals of 1, 10, 30 and 60 days of storage.Thermal transition analysis
Differential scanning calorimetry (DSC; Perkin Elmer instrument, DSC 800, France) was used to determine the thermal transition of the DHA nanoemulsions, DHA algae oil and Tween-40. The test sample of about 8.5–9.5 mg was placed in an aluminum pan and hermetically sealed before placing into the calorimeter thermocouples. The samples were then cooled from 25 °C to −60 °C with an increase of 5 °C min−1 and heated up to 60 °C simultaneously in the same process.Fourier transform infrared (FTIR) spectroscopy
The DHA nanoemulsions were analyzed via Fourier transform infrared (FTIR) spectroscopy (Nicolet 5700, M/S. Thermoelectron Corporation, Round Rock, TX). Similarly, DHA algae oil (control) and Tween-40 emulsifier were also analyzed. This study was performed at different time intervals (1st, 10th, 20th and 30th day). Pure FTIR-KBr was used for sample preparation and the scanning range was fixed at 500–4000 cm−1. In addition, 32 scans were used for all the samples. Initially, FTIR-KBr crystals were gently ground to make a fine powder. Subsequently, 80 μL of nanoemulsion was added to the KBr fine powder and mixed thoroughly. Furthermore, this mixture was pelletized using a pellet making kit and the obtained pellet was transparent in nature.Oxidative stability studies of DHA nanoemulsion
The lipid oxidative stability of the DHA nanoemulsions and the control, DHA algae oil (unencapsulated) was determined over the storage period of 30 days. 2 mL of emulsion was taken from capped test tubes. Thiobarbituric acid-reactive substances (TBARS) were used to measure the lipid oxidation reaction products. A solution of TCA (trichloroacetic acid)–TBA–HCl was prepared by mixing 75 g of TCA, 1.68 g of TBA, 8.8 mL of 12 M HCl, and 414 g of H2O. 100 mL of TCA–TBA–HCl solution was mixed with 3 mL of 2% (w/w) butylated hydroxytoluene in ethanol, and 2 mL of this solution was mixed with the same amount of emulsion sample. This solution mixture was vortexed and heated in a boiling water bath for 15 min. Furthermore, it was cooled down to room temperature using tap water for 10 min and centrifuged at 1000g for 10 min. After 10 min, the absorbance was measured at 532 nm using a UV-VIS spectrophotometer (UV-1700 Pharma Spec, SHIMADZU Corporation). TBARS concentrations were measured from the standard curve plotted using 1,1,3,3-tetraethoxypropane.14Determination of fatty acid composition
Fatty acid composition was determined using a gas chromatograph (Shimadzu 2010 plus system, Japan) fitted with a flame ionization detector (FID) and an RTX-2330 capillary column (30 m, 0.25 μm internal diameter and df of 0.20 μm). Helium was used as the carrier gas at a constant linear velocity of 20 cm s−1. The temperatures of the injector and FID detector were maintained at 240 and 250 °C, respectively. The oven temperature was initially held at 160 °C and increased to 250 °C at 5 °C min−1 and held for 3 min. Fatty acid methyl esters (FAME) were prepared using the procedure of Christie.15 Briefly, the extracted lipid was added in 0.2 mL of methanolic NaOH (2 N) solution and 1 mL of hexane. The reaction mixtures were vortexed for 15 s at room temperature and incubated in a water bath for 30 min at 50 °C. Furthermore, 0.2 mL of methanolic HCl (2 N) was added to the above mentioned mixture and vortexed for 15 s. This mixture was incubated in a water bath for 15 min at 50 °C, and then the upper layer was removed. Subsequently, the solvent was evaporated using nitrogen gas in the screw capped test tube.Determination of volatile compounds
Gas chromatography-mass spectrometry (GC-MS) analyses were performed using a Perkin Elmer instrument. Helium was used as the carrier gas at a constant linear velocity of 20 cm s−1. The temperature of the injector and the FID detector were maintained at 240 and 250 °C. The oven temperature was initially maintained at 150 °C and increased to 5 °C min−1 to 220 °C for 3 min. Electron impact mass spectra were obtained in the range of 40–400 m/z at 0.2 scans per s and the mode was kept as EI+. Volatiles were determined using both MS library searches and the comparison of retention times with the control. Moreover, the external standard of hexanal was also used to identify the aldehyde.NMR
DHA algae oil was extracted from the nanoemulsified DHA using a solvent mixture of chloroform and methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio). Subsequently, the extracted DHA structural features were studied and compared with the control DHA algae oil and Tween-40 emulsifier. A DHA methyl ester standard was used as the reference to identify the DHA peak in the algal oil NMR spectra. The samples (liquid form) were dissolved in deuterated chloroform for 1H and 13C NMR spectral studies. The spectral studies were carried out on a 500 MHz NMR spectrometer (Avance AQS 500, Bruker, Germany).
3 ratio). Subsequently, the extracted DHA structural features were studied and compared with the control DHA algae oil and Tween-40 emulsifier. A DHA methyl ester standard was used as the reference to identify the DHA peak in the algal oil NMR spectra. The samples (liquid form) were dissolved in deuterated chloroform for 1H and 13C NMR spectral studies. The spectral studies were carried out on a 500 MHz NMR spectrometer (Avance AQS 500, Bruker, Germany).
Statistical analysis
Results are expressed as the mean value ± SD of three or two independent experiments. Statistical analysis was carried out via the analysis of variance (ANOVA) using the SPSS statistical software version 16. Comparison of means was performed via Turkey's test. Furthermore, paired-sample for means t-test was performed for the creaming and oxidation stability studies using the Microsoft Office Excel 2010 software. The level of significance used was p < 0.05 for the entire statistical test.Results and discussion
Particle size distribution and morphology of the nanoemulsions
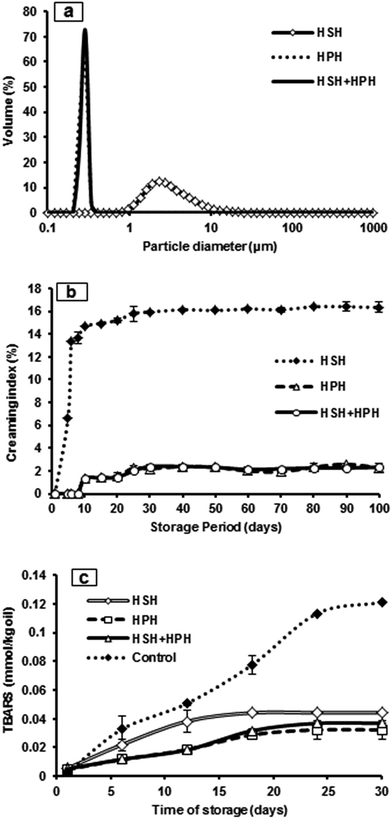
Particle size distribution is one of the essential parameters of food emulsions, which interlinks physical properties such as color, viscosity, texture and shelf life.16 Fig. 1a shows the droplet size distribution and polydispersity index (PDI) of the DHA nanoemulsions. The HPH and combination (HSH + HPH) techniques yielded a lower mean particle diameter of 11.17 and 11.31 nm, respectively, whereas HSH produced an 87 nm particle diameter. Shear force and turbulence are the high pressure emulsification factors, which contribute to the lower particle size in the HPH and HSH + HPH nanoemulsions. During the high pressure homogenization process (HPH and HSH + HPH), the particle size is reduced and their distribution is considerably altered. The smaller mean particle size offers a higher surface area and this improves physical stability due to its unique morphological and functional characteristics. Similarly, the polydispersity index of the HPH and combination techniques also depicted a lower value (0.348 and 0.303, respectively), which indicates that the particle size distributions were monomodal and a narrower PDI than HSH (0.516) is obtained. TEM images of the DHA nanoemulsions (HSH, HPH and HSH + HPH) are shown in Fig. 2a–f. All the emulsions showed a spherical shape with a droplet diameter in the nano-scale. Therefore, the morphology resulting from the HSH technique exhibited slightly larger sized droplets than the HPH and HSH + HPH, which highly correlates with the results obtained from the particle size distribution. Similarly, the nanoemulsion morphology obtained from confocal microscopy also confirmed spherical shaped droplets with a uniform size distribution in the nanometric range (Fig. 2i–iii). | ||
| Fig. 2 Transmission electron microscopy (TEM) and confocal laser scanning microscopy images of the DHA nanoemulsions. | ||
During the storage period of 100 days, there was an increase in mean particle size observed in all the nanoemulsions stored at different temperatures (Fig. 3 and 4a). The mean particle diameter of the HPH and HSH + HPH techniques was in the range of 238–258 nm for all the storage conditions. Moreover, they resulted in a narrow and uniform particle size distribution (Fig. 4a). On the contrary, the HSH emulsion exhibited micrometer sized (2.55–41.54 μm) particles for all the storage conditions. The oven stored emulsion showed a higher mean droplet diameter (41.54 μm) than the emulsion stored at other temperatures. The destabilization of the emulsions is related to the different forces acting on the emulsion system such as gravitational forces, inter-particle repulsive forces, attractive forces, flow forces, and molecular forces.17
 | ||
| Fig. 3 Physical stability studies of the DHA nanoemulsions over the storage period of 100 days at different temperature conditions. Scale bar represents 0.39 μm for all the emulsions. | ||
 | ||
| Fig. 4 Storage stability studies of the DHA nanoemulsions stored at room condition (28 ± 1 °C): (a) particle size distribution on 100th day, (b) creaming and (c) oxidative study. | ||
Moreover, a uniform nanometer droplet size distribution was found in the samples stored at 4 ± 1 °C and 28 ± 1 °C, whereas a non-uniform droplet size distribution was observed for the sample stored at 40 ± 0.2 °C. Therefore, a higher temperature with longer storage period influences the instability of the emulsions. At higher temperatures, there may be an impact on the properties of the emulsifier, which is adsorbed on the surface of the oil droplets. Moreover, it affects the emulsion physiochemical properties and stability.18 The mean particle diameter increased with increase in storage temperature and this effect was attributed to droplet aggregation. The growth of the emulsion droplet size was slower for the emulsion stored at a lower temperature than the emulsions stored at higher storage temperatures. When an emulsion is stored at lower temperatures molecular movement is reduced subsequently.19 Moreover, it is also due to the rate and frequency of inter-droplet collision that reduces the droplet growth effectively.20 Adjonu et al. reported that emulsions stored at higher temperature exhibit larger droplet sizes with multimodal size distributions than emulsions stored at lower temperatures.21
Particle charge characteristics of DHA nanoemulsion
A comparison of the zeta-potential for the HSH, HPH and combination (HSH + HPH) produced DHA nanoemulsions are shown in Fig. 1a. In colloidal dispersion system, the zeta potential may determine is overall physiochemical stability due to its electrical charge functions and their interactions.22 In this study, the zeta potential value of the HSH, HPH and HSH + HPH nanoemulsions were found to be low negative values. HPH and the combination of HSH + HPH resulted in a zeta-potential of −30.2 and −31.1 mV, respectively, whereas HSH yielded −25.2 mV which leads to instability in the later stage. Previous researchers have stated that the zeta potential value of dispersion systems should be around −30 mV to prevent instability in terms of aggregation and coalescence.23,24 Furthermore, it was reported that increase in surface charge can effectively prevent emulsion instability,25 which is due to the fact that surface charge leads to electrostatic repulsive force between emulsion droplets.26 The HSH nanoemulsion showed higher instability under all the storage conditions. Furthermore, this instability phenomenon was confirmed with storage stability studies on the emulsion morphology (Fig. 3). Thus, the combination technique showed a higher negative zeta-potential, which helps to increase the emulsion stability from environmental factors.Rheological characteristics
The rheological characteristics of the DHA nanoemulsions were studied via shear stress as a function of shear rate. Fig. 1b depicts the rheological behavior of the nanoemulsion prepared using the HSH, HPH and combination techniques. The high pressure treated techniques (HPH and HSH + HPH) exhibit similar rheological behavior when shear rate (s−1) versus shear stress increases. The presence of a linear relation between shear stress and shear rate in all the emulsification techniques shows that the nanoemulsions exhibited a Newtonian flow behavior. By varying the emulsification technique, the rheological behavior of the nanoemulsions was affected. Emulsion stability and rheological behavior are highly subjected to the interactions between oil droplets and the interfacial layer of oil/water in the emulsion system.27 Thus, the high pressure treated nanoemulsions were stable for longer time against flocculation, creaming and coalescence due to their rheological behavior and this was confirmed by storage stability studies (Fig. 1b).Stability of DHA nanoemulsions using different emulsification techniques
The storage stabilities of the DHA nanoemulsions (HSH, HPH and HSH + HPH) were analyzed via creaming stability, phase separation, centrifugation, flocculation, sedimentation, coalescence, and oiling off with different storage conditions for 100 days and the results are shown in Table 1 and Fig. 3. The color stability was done for 60 days under similar storage conditions.| Storage period (days) and samples | Phase separation | Centrifugation | Sedimentation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 ± 1 °C | 28 ± 1 °C | 40 ± 0.2 °C | 4 ± 1 °C | 28 ± 1 °C | 40 ± 0.2 °C | 4 ± 1 °C | 28 ± 1 °C | 40 ± 0.2 °C | ||
| a (−) no change; (+) slight change; (++) more change. | ||||||||||
| 1–8 | HSH | − | − | − | − | − | − | − | − | − |
| HPH | − | − | − | − | − | − | − | − | − | |
| HSH + HPH | − | − | − | − | − | − | − | − | − | |
| 20 | HSH | − | + | + | − | + | + | − | − | − |
| HPH | − | − | − | − | − | − | − | − | − | |
| HSH + HPH | − | − | − | − | − | − | − | − | − | |
| 40 | HSH | ++ | ++ | ++ | ++ | ++ | ++ | − | − | − |
| HPH | + | + | + | + | + | + | − | − | − | |
| HSH + HPH | + | + | + | + | + | + | − | − | − | |
| 100 | HSH | ++ | ++ | ++ | ++ | ++ | ++ | − | + | ++ |
| HPH | + | + | + | + | + | + | − | + | + | |
| HSH + HPH | + | + | + | + | + | + | − | + | + | |
Creaming stability
Creaming is one of the emulsion instability mechanisms that are formed by gravitational separation. The creaming stability of the DHA nanoemulsions was analyzed under different storage conditions such as refrigeration (4 ± 0.1 °C), room (28 ± 1 °C; Fig. 4b) and oven (40 ± 0.2 °C) over the storage period of 100 days. The HPH and HSH + HPH emulsion technique showed no cream formation in all the conditions during storage for 8 days. However, a slight change was observed in the emulsion stored for 10 days in all the stored conditions. On the other hand, the HSH nanoemulsion was found to be stable for only 4 days and after that creaming increased rapidly. This is because of the fact that the emulsions prepared via HSH yielded an uneven droplet size distribution and higher mean particle size. An increase in emulsion droplet collision and aggregation can increase the influence for the droplet to cream.21 Moreover, the HSH emulsion showed a 14 fold increase at refrigeration (showed in ESI†) and room temperature (Fig. 4b), whereas a 16 fold increase was observed from the emulsion stored at oven temperature (showed in the ESI†). There was a large variation (P < 0.05) between the HSH and high pressure treated techniques. However, there was no significant difference observed between the HPH and HSH + HPH emulsions stored at all the temperatures. During the high pressure homogenization process, a decrease in average fat droplets size was achieved (11.17 to 11.31 nm), which helps to reduce the creaming velocity (as per Stokes' law) and increases the emulsion stability significantly,28 whereas an increase in average mean particle size and higher PDI value (0.516) were found in the HSH emulsion. In addition, a greater droplet–droplet repulsive force acts on the emulsion prepared via high pressure homogenization due to presence of a lower negative charge (Fig. 1b). Moreover, it showed no further increase in creaming at all the stored conditions and it exhibited higher storage stability over the period of 100 days (Fig. 3).Phase separation, centrifugation and sedimentation stability
The initial instability of fine triglyceride oil in water emulsion is due to creaming, which eventually influences macroscopic phase separation into separate visible regions of cream and serum.29 In this study, the overall instability was not observed until eight days of storage for all the emulsions at different conditions. However, slight phase separation was observed in the HSH nanoemulsion under the storage of oven and room conditions. In contrast, the HPH and HSH + HPH emulsions showed no changes in all the stored conditions until the 20th day. Subsequently, there was a change in HPH and HSH + HPH on the 40th day in all the stored conditions. Another study on phase separation (without centrifugation) also showed no changes until the 20th day for the HPH and HSH + HPH emulsion under all the conditions. Furthermore, slight changes were observed during the 40th day of storage, which is similar to the centrifugation study. This may be due to the change in temperature and increase in droplet size of the emulsions. On the contrary, the sedimentation study did not show any instability until 40 days (Table 1 and Fig. 3) and more changes were found in the HSH emulsion over the storage of 100 days for the oven temperature. This is because, when there is an increase in storage temperature, the mean particle diameter increases considerably and this directly influences droplet growth (as explained earlier). Due to the increase in droplet diameter, the density of droplets was increased and this influences gravitational separation. However, interestingly there was no sedimentation observed in all the emulsions stored under refrigerated conditions. This implies that the DHA nanoemulsions stored at the lower temperature showed excellent stability towards gravitational separation.Flocculation, coalescence and oiling off stability
Flocculation was not observed in the emulsions prepared via the HPH and HSH + HPH techniques over the storage period of 100 days at all the stored temperatures (Fig. 3). However, flocculation was observed in the HSH nanoemulsion stored at refrigerated and room temperature. The flocculation of an emulsion is influenced by van der Waals attractions and it occurs due to the insufficient repulsive energy between oil droplets.30 Moreover, during the storage period, the oven stored nanoemulsion showed more coalescence. This is because, the small flocculated drops coalesced into larger drops in presence of elevated heat and prolonged storage.31 In addition, thinning and disruption of the liquid film between the oil droplets caused coalescence, which also influenced some oil separation in the later stage due to the ultimate joining of droplets in the emulsion system.32 Therefore, the decrease in storage temperature influences the increase in nanoemulsion stability towards droplet coalescence.7During the coalescence process, large droplets are formed that eventually influences oiling off. Moreover, in an emulsion system, coalescence and oiling-off can happen due to the deformation of oil droplets through external stresses acting on the interfacial layers of oil droplets.33 In this study, 4% of oiling off was observed in the HSH emulsion stored under oven conditions on the 100th day and further this was confirmed by observing the morphology of the emulsion (Fig. 3). This instability mechanism is also known as flow-induced coalescence, which comprises friction between the emulsion droplets, wherein droplets merge together by capillary pressure. Beyond the critical pressure, the frictional force between the oil droplets cannot relax by slipping between the droplet surfaces. Therefore, this leads to stretching and rupturing of the thin film present on the oil droplet surface that originated from oiling-off.33 Moreover, oiling off may occur due to the influence of the higher storage temperature, emulsification technique and prolonged storage of the emulsion. However, there was no oiling off found in the HPH and HSH + HPH emulsion. In the case of the HSH treatment, the droplets in this nanoemulsion coalesced due to the interfacial layer of the systems, which was more flexible and liable to disruption.34 Therefore, the oven stored HSH emulsion showed three different layers, which were the separation of DHA algae oil with an opaque cream at the top, turbid with milky emulsion at the middle and more sedimentation at the bottom (Fig. 3). Thus, these studies suggest that the heating of an emulsion influences an increase in mean particle diameter, droplet flocculation, coalescence, phase separation, sedimentation, creaming and oiling off.
Color stability
There were no color differences observed in the DHA emulsion at all the storage conditions until 10 days (showed in ESI†). However, slight changes began to be observed in the HSH produced emulsion over the storage of 10 days in all the temperature conditions. This is because of the instability produced by the high speed homogenization technique and lower stability at elevated storage temperatures. Karthik and Anandharamakrishnan also reported that changes in storage temperature influence the color instability of emulsions and this was studied using a DHA algae oil emulsion.12 Therefore, the refrigerated HSH sample showed slight instability, whereas more instability was observed in the emulsions stored at 28 ± 1 °C and 40 ± 0.2 °C temperature for 60 days, which confirms the higher level of the b* (yellowness) value. There was no change in color difference observed in the high pressure treated emulsion during the storage stability studies. These nanoemulsions had a higher L* value that showed the strength of white color domination, but not much variation in a* and b* values.Thermal transition
The DSC melting thermograms of DHA algae oil, Tween-40 and nanoemulsified DHA are shown in Fig. 5a and b. DHA algae oil showed a broad melting thermopeak (Tm) at −12.05 °C and one sharp peak at −1 °C. The Tween-40 melting temperature was found at 23.27 °C. The transition enthalpy (ΔH) values of DHA and Tween-40 were found to be 16.04 J g−1 and 26.01 J g−1, respectively (Fig. 5a). The nanoemulsified DHA melting peak was compared with respect to the HSH, HPH and combination techniques. The melting peak of the HSH, HPH and HSH + HPH nanoemulsified DHA were observed at 3.95 °C, 4.01 °C and 3.66 °C, respectively (Fig. 5b). The transition enthalpy of the HPH treated DHA nanoemulsion was found to be at the higher value of 282.03 J g−1 (HSH + HPH) and 281.39 J g−1 (HPH). On contrary, the lower ΔH value of 275.90 J g−1 was observed for the HSH nanoemulsion. This may be due to the fact that the number of oil droplets present is more per gram of HPH treated nanoemulsion than HSH emulsion. Moreover, the transition temperature and enthalpy change influenced the effect of emulsifier concentration for the emulsion system.35 The DHA algae oil showed vast melting temperature differences and melting shift when compared to all the nanoemulsified DHA. Kabri et al. reported that lipid nanoemulsion has higher melting temperature when compared to oil melting temperature.36 Moreover, this obtained DHA nanoemulsions thermogram clearly indicated that there was a protecting layer that acts on the oil droplets to extend the melting temperature of oil. Moreover, lower oil droplet sizes exhibited lower melting reaction in emulsions.37 In addition, the result was clear that after nanoemulsification, DHA was effectively protected from environmental conditions and this extended the shelf life. | ||
| Fig. 5 DSC melting thermograms: (a) DHA and Tween-40; (b) different high energy emulsification techniques. | ||
Fourier transform infrared (FTIR) spectroscopy
FTIR analysis was performed to examine the stability of DHA present in all the nanoemulsified samples. Fig. 6 shows the FTIR spectra of DHA, Tween-40 and nanoemulsified DHA (HSH, HPH and HSH + HPH techniques). The intense absorption at 3014.3 cm−1 represents the –C–H stretching vibration of the cis double bond of unsaturated fatty acids. However, this observed peak confirms the presence of DHA (control-DHA algae oil) in all the nanoemulsions. Karthik and Anandharamakrishnan also observed a similar trend in their DHA FTIR spectra.12 The bands absorbed at 2926.8 and 2854.8 cm−1 denote the asymmetric and symmetric stretching vibration of aliphatic –CH2 fatty acid hydrocarbon chains.38 The very strong ester carbonyl (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) functional groups observed at 1751.3 cm−1 indicate the stretching bands of triglyceride. A weak absorption peak was found at 1651.9 cm−1, which relates the characteristics of the –C
O) functional groups observed at 1751.3 cm−1 indicate the stretching bands of triglyceride. A weak absorption peak was found at 1651.9 cm−1, which relates the characteristics of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– stretching vibration of cis olefins. The absorption at 1463.7 cm−1 indicates the medium bending vibrations of the –C–H (CH2 and CH3) aliphatic functional group. The peak observed at 1371.3 cm−1 belongs to the symmetric bending band of the –C–H (CH3) group. Furthermore, the very strong stretching and bending vibration of the –C–O and –CH2 groups were observed at 1149.3 cm−1.39 Watanabe et al. reported the observation of a broad band at 719 cm−1, but not in the range of 960–980 cm−1, which confirmed that the fatty acid was cis double bonds and not trans double bonds.40 These results clearly demonstrate that the fatty acid was cis-4,7,10,13,16,19-DHA. In addition, the concentration of DHA was very low in the nanoemulsions (8 μL of oil in 80 μL of emulsion). Therefore, the peaks corresponding to –(CH2)n– and –HC
C– stretching vibration of cis olefins. The absorption at 1463.7 cm−1 indicates the medium bending vibrations of the –C–H (CH2 and CH3) aliphatic functional group. The peak observed at 1371.3 cm−1 belongs to the symmetric bending band of the –C–H (CH3) group. Furthermore, the very strong stretching and bending vibration of the –C–O and –CH2 groups were observed at 1149.3 cm−1.39 Watanabe et al. reported the observation of a broad band at 719 cm−1, but not in the range of 960–980 cm−1, which confirmed that the fatty acid was cis double bonds and not trans double bonds.40 These results clearly demonstrate that the fatty acid was cis-4,7,10,13,16,19-DHA. In addition, the concentration of DHA was very low in the nanoemulsions (8 μL of oil in 80 μL of emulsion). Therefore, the peaks corresponding to –(CH2)n– and –HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– (cis) were not pronounced at 719 cm−1. The Tween-40 emulsifier used in the encapsulation of DHA showed almost similar FTIR spectra as compared to the control DHA algae oil. These results clearly indicate that there was no chemical interaction between the oil and emulsifier. Moreover, the 30 days stored emulsion samples at different conditions also did not show any major differences when compared to the control DHA (data showed in ESI† section).
CH– (cis) were not pronounced at 719 cm−1. The Tween-40 emulsifier used in the encapsulation of DHA showed almost similar FTIR spectra as compared to the control DHA algae oil. These results clearly indicate that there was no chemical interaction between the oil and emulsifier. Moreover, the 30 days stored emulsion samples at different conditions also did not show any major differences when compared to the control DHA (data showed in ESI† section).
Oxidative stability of DHA nanoemulsion
Oxidative stability studies of the nanoemulsified DHA oil and control were conducted under different storage conditions. Fig. 4c depicts the oxidative stability of the DHA nanoemulsions (stored at 28 ± 1 °C) and this was compared with the different emulsification techniques (HSH, HPH and HSH + HPH) and control during the storage period of 30 days. Oxidative stability was measured using the TBARS method, which is an identifier of lipid oxidation. Lipid oxidation was monitored at different time intervals and there was no significant difference observed between the HPH and HSH + HPH emulsions at the initial day. However, there was a significant difference (P < 0.05) observed when compare to the HSH emulsion. Therefore, when compared to the control DHA, the nanoemulsified DHA showed slightly more oxidation on the first day of storage. This may be due to the emulsification process, which involves high pressure processing. However, during the storage period, control DHA showed an increase in oxidation at all the temperature conditions. Furthermore, it was found that the control DHA experienced higher oxidation than of the nanoemulsified DHA (P < 0.05).Oxidation of all the stored emulsions increased slowly, whereas slight differences were observed among the samples kept under refrigeration conditions (shown in the ESI†). The HSH emulsion showed slightly lower oxidation when compared to the high pressure treated emulsion but later on the oxidation was close to the HPH and HSH + HPH emulsion. In addition, the oxidation of the HSH DHA nanoemulsion increased linearly as compared to the HPH and HSH + HPH emulsions during storage at room conditions. This clearly indicates the important role of the emulsification techniques and storage conditions. On the other hand, the oven stored emulsions showed slightly lower oxidation in the HSH + HPH emulsions than the HSH emulsions. Moreover, the oxidation value was found to be comparatively less in the HPH DHA emulsion. Among all the emulsification techniques, the high pressure treated emulsions (HPH and HSH + HPH) resulted in a lower oxidative value for both room and oven conditions. In this study, the high pressure processed nanoemulsions showed higher thermal stability and less oxidation than the control. Thus, this result strongly reinforces the advantages of the high pressure emulsification process.
Determination of fatty acid composition and volatile compounds
The overall fatty acid compositions and the result from DHA algae oil (bulk oil) were compared with the HSH, HPH and HSH + HPH nanoemulsions (shown in the ESI†). DHA algae oil showed 38.11% ± 0.07% of DHA from the total fatty acid composition. In this study, an almost similar percentage of DHA fatty acid composition was measured in all the nanoemulsions. Moreover, there was no change in major fatty acid compositions in all the samples. These results clearly indicate that during the emulsification process, there was no loss in DHA and it showed stability against the emulsification process. During the emulsification process high turbulent force was applied to make very tiny oil droplets, which might have influenced polyunsaturated oils to be thermodynamically unstable. However, in the present study, it did not show any changes in DHA fatty acid. The presence of aldehyde compounds was analyzed in the HSH, HPH and HSH + HPH DHA nanoemulsion using GC-MS (figure shown in the ESI†). There was no degradation compound observed after the formation of the DHA nanoemulsions, which was confirmed with hexanal standards. The retention time (RT) of 3.98 min was observed for hexanal and this RT was compared with nanoemulsified DHA. From this study, it was confirmed that there was no peak found at this particular RT. Furthermore, the fatty acids profile spectra were verified by mass spectrometry. These obtained results highlight that after the emulsification process, DHA shows stability against chemical instability.NMR
The structural characteristics of DHA algae oil extracted from the nanoemulsions (HSH, HPH and HSH + HPH) were examined using 1H NMR and 13C NMR. In the present study, NMR was used to investigate the effect of the emulsification technique to improve the stability of DHA. The NMR spectra of DHA algae oil (control), DHA methyl ester (standard) and Tween-40 emulsifier (data shown in the ESI†) were compared with the DHA algae oil extracted from the nanoemulsions in terms of chemical shift and integration values (Fig. 7a–k). In general, algae oil includes other fatty acid groups (e.g. myristic acid, palmitic acid, stearic acid, and docosapentaenoic acid) along with DHA fatty acid. Therefore, a DHA methyl ester (standard, Fig. 7a, b and g) NMR spectrum was used to identify the specific DHA NMR peaks from the group of fatty acid compositions, whereas the terminal methyl group integral of DHA was calibrated to the value of 3 at 0.99 ppm. Because other fatty acids characteristic peaks are merged with DHA, it was not feasible to separate them in the olefinic as well as aliphatic region. | ||
| Fig. 7 1H NMR (a–f) and 13C NMR (g–k) spectra of DHA algae oil, DHA methyl ester (standard) and different high energy techniques nanoemulsions. | ||
Based on the NMR data analysis, 12 olefinic protons of DHA were present in the range of 5.32–5.45 as multiplets. 10 methylene protons, which had the chemical shift of 2.82 to 2.89, were observed as multiplets. Four methylenic protons next to the carboxylic acid, which had the chemical shift in the range of 2.37–2.43, were integrated to four protons. Furthermore, two methylenic protons next to the terminal methyl and three terminal methyl protons were observed at 2.09 (pentet) and 0.99 (triplet), respectively. The proton NMR spectrum of the control DHA was compared with that of DHA extracted from the nanoemulsion to identify any structural changes. It was observed that the NMR peaks of the DHA algae oil were comparable to the standard DHA. Moreover, the NMR peaks of the DHA algae oil were integrated at the same δ value as that of the standard DHA. Furthermore, these proton NMR spectra confirm that there was no change in the structure of DHA.
The 13C NMR spectrum presents a terminal methyl signal at 13.92 ppm and two carbon atoms next to the terminal methyl group, which are present at 20.23 and 33.77 ppm. A bunch of methylenes were observed at 25.22, 22.25 and 25.319 ppm. Moreover, all the olefins were observed between 125 ppm and 132 ppm and also one carbonyl carbon signal was observed at 173 ppm. From the NMR spectral results it is evident that molecular structure of DHA algae oil did not changed even after the emulsification process and this was confirmed with the spectra of standard DHA. In addition, it was found that DHA did not chemically react with the Tween-40 emulsifier during the nanoemulsification process. Therefore, the NMR study confirms that there was no change in structural behavior after the formation of the DHA nanoemulsions. This is because the encapsulation DHA in very tiny molecules prevented the structure of DHA from several environmental factors.
Conclusions
DHA nanoemulsions were prepared via high energy emulsification techniques (HSH, HPH and HSH + HPH) and their physicochemical properties were studied to compare their stabilities. The HPH and HSH + HPH nanoemulsions yielded a lower mean particle diameter than HSH. Similarly, HPH and the combination of HSH + HPH depicted a higher negative charge, whereas HSH showed a lower negative charge that influences emulsion instability during the storage period. After studying their properties up to 100 days at different storage temperatures, it is clearly indicated that the HPH involved emulsification process (HPH and HSH + HPH) produces stable DHA nanoemulsions in terms of lower particle size, morphology and other physical properties. There was no change in fatty acid profile and structural changes of DHA in any of the emulsions. The refrigerated HPH and HSH + HPH DHA exhibited lower lipid oxidation than the emulsion stored at other conditions (28 ± 1 °C and 40 ± 0.2 °C). In this study, the emulsion prepared via the high pressure and combination technique exhibited higher stability in terms of physicochemical properties. On the contrary, thermal transition of the nanoemulsion results in higher stability towards HSH + HPH than the others. However, amongst the high energy techniques, better stability was achieved via HSH + HPH technique compared to HPH. Thus, the prepared DHA nutrient delivery system can be used in food and pharmaceutical industries to improve the stability and bioavailability of DHA.Acknowledgements
Authors wish to thank Prof. Ram Rajasekaran, Director of CSIR-CFTRI for the support and help. Author (PK) wish to thank the Council of Scientific and Industrial Research for awarding SRF fellowship. Authors also acknowledge the CSIR for the financial support through WELFO project (BSC-0202). We wish to thank Dr Radha and Mr Manjunatha, J. R. for their help in handling high pressure homogenizer and NMR, respectively. In addition, the authors are very grateful to Tamil Nadu Agricultural University, Dept. of Nano Science and Technology, Coimbatore for providing the Transmission Electron Microscopy facility.Notes and references
- D. Swanson, R. Block and S. A. Mousa, Adv Nutr: Int Rev J., 2012, 3, 1–7 CrossRef CAS PubMed
.
- S. Krauss-Etschmann, R. Shadid, C. Campoy, E. Hoster, H. Demmelmair, M. Jimenez and B. V. Koletzko, Am. J. Clin. Nutr., 2007, 85, 1392–1400 CAS
.
- I. Capek, Adv. Colloid Interface Sci., 2004, 107, 125–155 CrossRef CAS PubMed
.
- T. F. Tadros, P. Izquierdo, J. Esquena and C. Solans, Adv. Colloid Interface Sci., 2004, 108–109, 303–318 CrossRef CAS PubMed
.
- C. Anandharamakrishnan, Techniques for Nanoencapsulation of Food Ingredients, Springer, New York, 2014 Search PubMed
.
- E. Acosta, Curr. Opin. Colloid Interface Sci., 2009, 14, 3–15 CrossRef CAS
.
- P. Karthik, P. N. Ezhilarasi and C. Anandharamakrishnan, Crit. Rev. Food Sci. Nutr., 2015 DOI:10.1080/10408398.2015.1006767
.
- P. N. Ezhilarasi, P. Karthik, N. Chhanwal and C. Anandharamakrishnan, Food Bioprocess Technol., 2013, 6, 628–647 CrossRef CAS
.
- M. Herrera, Analytical techniques for studying the physical properties of lipid emulsions, Springer, 2012, vol. 3 Search PubMed
.
- D. J. McClements, Food Emulsions: Principles, Practices and Techniques, CRC Press, Boca Raton, FL, 2005 Search PubMed
.
- X. Huimin, L. Lin, G. Shilin, W. Elfalleh, H. Shenghua, S. Qinghai and M. Ying, Food Bioprocess Technol., 2014, 7, 567–574 CrossRef
.
- P. Karthik and C. Anandharamakrishnan, Food Bioprocess Technol., 2013, 6, 2780–2790 CrossRef CAS
.
- P. Thanasukarn, R. Pongsawatmanit and D. J. McClements, J. Agric. Food Chem., 2006, 54, 3591–3597 CrossRef CAS PubMed
.
- S. J. Lee, S. J. Choi, Y. Li, E. A. Decker and D. J. McClements, J. Agric. Food Chem., 2010, 59, 415–427 CrossRef PubMed
.
- W. W. Christie, Gas chromatography and lipids, The Oily Press, Ayr, Scotland, 1989 Search PubMed
.
- C. Huck-Iriart, V. M. P. Ruiz-Henestrosa, R. J. Candal and M. L. Herrera, Food Bioprocess Technol., 2013, 6, 2406–2418 CrossRef CAS
.
- A. Chiralt, Food emulsion, in Food Engineering, ed. G. V. Barbosa-Canovas, EOLSS, USA, 2009 Search PubMed
.
- B. M. Degner, C. Chung, V. Schlegel, R. Hutkins and D. J. McClements, Compr. Rev. Food Sci. Food Saf., 2014, 13, 98–113 CrossRef CAS
.
- L. C. B. Zuge, C. W. I. Haminiuk, G. M. Maciel, J. L. M. Silveira and A. de Paula Scheer, J. Food Eng., 2013, 116, 72–77 CrossRef CAS
.
- C. Freitas and R. H. Muller, Int. J. Pharm., 1998, 168, 221–229 CrossRef CAS
.
- R. Adjonu, G. Doran, P. Torley and S. Agboola, Food Hydrocolloids, 2014, 41, 169–177 CrossRef CAS
.
- F. Nielloud and G. Marti-Mestres, Pharmaceutical emulsions and suspensions, Marcel Dekker, New York, 2000 Search PubMed
.
- B. S. Chu, S. Ichikawa, S. Kanafusa and M. Nakajima, J. Agric. Food Chem., 2007, 55, 6754–6760 CrossRef CAS PubMed
.
- Z. Wang, M. A. Neves, L. J. Yin, I. Kobayashi, K. Uemura and M. Nakajima, Food Sci. Technol. Res., 2012, 18, 149–156 CrossRef CAS
.
- J. Stachurski and M. MichaLek, J. Colloid Interface Sci., 1996, 184, 433–436 CrossRef CAS PubMed
.
- W. Liu, D. Sun, C. Li, Q. Liu and J. Xu, J. Colloid Interface Sci., 2006, 303, 557–563 CrossRef CAS PubMed
.
- E. Dickinson, Colloids Surf., B, 1999, 15, 161–176 CrossRef CAS
.
- A. Desrumaux and J. Marcand, Int. J. Food Sci. Technol., 2002, 37, 263–269 CrossRef CAS
.
- E. Dickinson, Food Hydrocolloids, 2003, 17, 25–39 CrossRef CAS
.
- T. Tadros, Adv. Colloid Interface Sci., 2004, 108, 227–258 CrossRef PubMed
.
- G. Urbina-Villalba, A. Forgiarini, K. Rahn and A. Lozsan, Phys. Chem. Chem. Phys., 2009, 11, 11184–11195 RSC
.
- Modern Aspects of Emulsion Science, ed. B. P. Binks, Royal Society of Chemistry Publication, Cambridge, 1998 Search PubMed
.
- G. A. van Aken, T. B. J. Blijdenstein and N. E. Hotrum, Curr. Opin. Colloid Interface Sci., 2003, 8, 371–379 CrossRef CAS
.
- B. Ozturk, S. Argin, M. Ozilgen and D. J. McClements, J. Food Eng., 2014, 142, 57–63 CrossRef CAS
.
- J. Rao and D. J. McClements, J. Agric. Food Chem., 2011, 59, 5026–5035 CrossRef CAS PubMed
.
- T. H. Kabri, E. Arab-Tehrany, N. Belhaj and M. Linder, J. Nanobiotechnol., 2011, 9, 1–8 CrossRef PubMed
.
- R. Shukat, C. Bourgaux and P. Relkin, J. Therm. Anal. Calorim., 2012, 108, 153–161 CrossRef CAS
.
- R. M. Silverstein, G. C. Bassler and T. C. Morrill, Infrared spectrometry, in Spectrometric Identification of Organic Compounds, Wiley, New York, 5th edn, 1991, pp. 71–143 Search PubMed
.
- D. Wu and Y. He, Food Chem., 2014, 158, 93–100 CrossRef CAS PubMed
.
- K. Watanabe, C. Ishikawa, I. Ohtsuka, M. Kamata, M. Tomita, K. Yazawa and H. Muramatsu, Lipids, 1997, 32, 975–978 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra12876e |
| This journal is © The Royal Society of Chemistry 2016 |


