A reversible rhodamine 6G-based fluorescence turn-on probe for Fe3+ in water and its application in living cell imaging†
Yaming Liua,
Rong Shenb,
Jiaxi Rua,
Xiang Yaoa,
Yang Yanga,
Haile Liua,
Xiaoliang Tanga,
Decheng Baib,
Guolin Zhang*a and
Weisheng Liu*a
aKey Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, State Key Laboratory of Applied Organic Chemistry, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000, China. E-mail: zhanggl@lzu.edu.cn; liuws@lzu.edu.cn
bLaboratory Centre for Medical Science, School of Basic Medical Sciences, Lanzhou University, Key Lab of Preclinical Study for New Drugs of Gansu Province, Lanzhou 730000, P. R. China
First published on 23rd June 2015
Abstract
A reversible fluorescent probe L based on rhodamine 6G is synthesized for the optical detection of Fe3+ in water. This probe displays favorable selectivity for Fe3+ with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. It displays a fluorescence turn-on response in the process of rhodamine ring opening with Fe3+ and a fluorescence turn-off response with the addition of Na4P2O7 to the L-Fe3+ complex. The association constant between the probe and Fe3+ was detected to be 3.5 × 104 M−1, and the corresponding detection limit was calculated to be 1.9 × 10−8 M according to fluorescence titration analysis. Furthermore, bioimaging investigations indicated that this probe was cell permeable and suitable for monitoring intracellular Fe3+ in living cells by confocal microscopy.
1 stoichiometry. It displays a fluorescence turn-on response in the process of rhodamine ring opening with Fe3+ and a fluorescence turn-off response with the addition of Na4P2O7 to the L-Fe3+ complex. The association constant between the probe and Fe3+ was detected to be 3.5 × 104 M−1, and the corresponding detection limit was calculated to be 1.9 × 10−8 M according to fluorescence titration analysis. Furthermore, bioimaging investigations indicated that this probe was cell permeable and suitable for monitoring intracellular Fe3+ in living cells by confocal microscopy.
Introduction
Iron, as a metal nutrient required for life and the most abundant and versatile transition metal, is no doubt one of the most important because of its crucial role in biochemical process.1–3 In the human body, numerous enzymes use iron as a catalyst for electron transfer, oxygen metabolism, and both DNA and RNA syntheses.1–6 Indeed, both deficient iron and excessive iron can induce biologic disorders in the human body. Iron deficiency can lead to anemia, hypoimmunity and low blood pressure because it can cause difficulties in oxygen delivery to cells. On the other hand, excessive iron levels could be associated with the underlying mechanisms of many neurodegenerative diseases such as Parkinson's disease and Alzheimer's disease.7,8 Therefore, the development of methods for the rapid and accurate detection of Fe3+ in complicated aqueous environment or biological environments is an important goal.In past decades, conventional methods such as atomic absorption spectroscopy (AAS), colorimetry, and inductively coupled plasma atomic emission spectroscopy (ICP-AES) have been used for the detection of Fe3+.9–13 However, the methods mentioned above require sophisticated equipment, tedious sample preparation procedures and highly skilled personnel.
However, fluorescence methods that are widely used by researchers can avoid these shortcomings while maintaining the efficiency and accuracy of the traditional methods.14–16 Numerous fluorescent probes have been exploited for the selective detection of Fe3+ ions.17–25 Most early probes showed a fluorescence quenching (turn-off) response due to the paramagnetic nature of the Fe3+ ion, which limits their application in biological systems.26,27 The turn-on type signal is more superior than the turn-off type due to its better overall signal processing in biosystems.28 Spirocyclic derivatives of rhodamine and fluorescein dyes based on spiroring-opening processes can lead to a turn-on fluorescence change. Rhodamine-based fluorescence turn-on probes for Fe3+ ion detection have been reported during recent years.29–34 Unfortunately, only a few successful examples of fluorescent probes for the detection of Fe3+ ions in aqueous solutions or pure water have been established, thus hampering analytical application in real water samples and cells. In addition, these probes are generally irreversible due to their reaction-based nature.19,30 Therefore, fluorescence enhancement (turn-on) through the chelation of Fe3+ ions with any fluorophore is a challenging task in vitro as well as in vivo.
Herein, we report a novel, reversible fluorescence turn-on probe for the determination of Fe3+ ion in water with a mild pH ranging from 5 to 8. It displays a fluorescence turn-on response in the process of rhodamine ring opening with Fe3+ and a fluorescence turn-off response when adding Na4P2O7 to the L-Fe3+ complex (Scheme 1). Moreover, the biological experiments proved that L could be used for the fluorescence imaging of Fe3+ in living cells.
Experimental
General
All reagents and solvents were obtained commercially and used without further purification unless otherwise noted. Mass spectra (ESI) were obtained using a Bruker MicroTOF ESI-TOF mass spectrometer. 1H NMR and 13C NMR spectra were recorded using a JNM-ECS-400 MHz and referenced to the solvent signals. Absorption spectra were recorded using a Cary-5000 UV-Vis-NIR spectrophotometer. Fluorescence spectra were performed using a Hitachi F-7000 spectrofluorophotometer. The quantum efficiencies were determined through an absolute method using an integrating sphere on a FLS920 from Edinburgh Instruments. All pH measurements were made with a pH-10C digital pH meter.Synthesis of compound 1
As shown in Scheme 2, compound 1 was synthesized according to the reported procedure.35,36 1H NMR (400 MHz, CDCl3, δ ppm): 7.98–7.86 (m, 1H), 7.49–7.37 (m, 2H), 7.04 (d, J = 5.4, 2.7 Hz, 1H), 6.32 (s, 2H), 6.20 (s, 2H), 3.49 (t, J = 5.0 Hz, 2H), 3.23–3.16 (m, 4H), 3.14 (t, J = 6.7 Hz, 2H), 2.33 (t, J = 6.7 Hz, 2H), 1.88 (s, 6H), 1.30 (t, J = 7.1 Hz, 6H). 13C NMR (100 MHz, CDCl3, δ ppm): 168.62, 153.65, 151.89, 147.78, 132.54, 131.36, 128.38, 128.14, 123.73, 122.85, 118.44, 118.16, 106.12, 96.73, 65.05, 43.92, 40.69, 38.64, 16.63, 14.88. ESI-MS m/z: calcd for C28H32N4O2 456.2525, found: 457.3306 [M + H]+.Synthesis of compound L
A mixture of compound 1 (0.912 g, 2 mmol) and 4-dimethylaminopyridine (DMAP 0.012 g, 0.1 mmol) was taken in 20 mL of N,N-dimethylformamide (DMF).37,38 The resultant solution was heated under reflux conditions and stirred for 8 hours. Upon the completion of the reaction, which is monitored by TLC, there is a large amount of precipitate when too much water is added. The crude product was purified with a silica gel column using a mixture of dichloromethane and methanol (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). Yield: 0.647 g (71%), white powder. 1H NMR (400 MHz, CDCl3, δ ppm): 7.97 (s, 1H), 7.94–7.85 (m, 1H), 7.51–7.40 (m, 2H), 7.11–7.00 (m, 1H), 6.32 (s, 2H), 6.18 (s, 2H), 3.53 (s, 2H), 3.32–3.24 (m, 2H), 3.19 (q, J = 7.1 Hz, 4H), 2.97 (d, J = 9.7, 4.5 Hz, 2H), 1.87 (s, 6H), 1.30 (t, J = 7.1 Hz, 6H). 13C NMR (100 MHz, CDCl3, δ ppm): 170.01, 161.16, 153.74, 151.74, 147.71, 133.06, 130.49, 128.39, 128.00, 124.03, 122.89, 118.31, 105.06, 96.61, 65.79, 39.47, 39.34, 38.39, 16.83, 14.77. ESI-MS m/z: calcd for C29H32N4O3 484.2474, found: 485.2648 [M + H]+.
1, v/v). Yield: 0.647 g (71%), white powder. 1H NMR (400 MHz, CDCl3, δ ppm): 7.97 (s, 1H), 7.94–7.85 (m, 1H), 7.51–7.40 (m, 2H), 7.11–7.00 (m, 1H), 6.32 (s, 2H), 6.18 (s, 2H), 3.53 (s, 2H), 3.32–3.24 (m, 2H), 3.19 (q, J = 7.1 Hz, 4H), 2.97 (d, J = 9.7, 4.5 Hz, 2H), 1.87 (s, 6H), 1.30 (t, J = 7.1 Hz, 6H). 13C NMR (100 MHz, CDCl3, δ ppm): 170.01, 161.16, 153.74, 151.74, 147.71, 133.06, 130.49, 128.39, 128.00, 124.03, 122.89, 118.31, 105.06, 96.61, 65.79, 39.47, 39.34, 38.39, 16.83, 14.77. ESI-MS m/z: calcd for C29H32N4O3 484.2474, found: 485.2648 [M + H]+.
General spectroscopic procedures
Stock solutions (10 mM) of perchlorate salts (Na+, K+, Li+, Ca2+, Mg2+, Mn2+, Cu2+, Zn2+, Cr3+, Hg2+, Ag+, Cd2+, Ni2+, Co2+, Pb2+, and Fe3+), Fe2SO4 and all lanthanide ions except for Pm (radioactive elements) were prepared. Water solutions of ethanedioic acid, EDTA, tartaric acid and propanedioic acid were also prepared at the same concentration. The probe L stock solution (10 mM) was also prepared in dimethyl sulfoxide (DMSO). Test solutions were prepared by placing a calculated amount of L stock solution into a test tube with 2.0 mL distilled water and adding an appropriate aliquot of each metal stock. All spectra were recorded 0.5 h after the adding and mixing at 25 °C with the excitation wavelength set at 500 nm (excitation/emission slit widths: 2.5 nm).Cell culture and confocal fluorescence imaging
HBL-100 cells were maintained in a DMEM medium supplemented with 10% FBS (fetal bovine serum), 100 units per mL penicillin, and 100 μg mL−1 streptomycin at 37 °C under a humid atmosphere containing 5% CO2. Cells (5 × 108 L−1) were plated on 18 mm glass coverslips and allowed to adhere for 24 h. Then, they were treated with L (10 μM) and incubated for 30 min. Subsequently, the cells were treated with 100 μM Fe (ClO4)3·6H2O. Cells were incubated for 30 min and washed three times with PBS to remove free compounds and ions before analysis. HBL-100 cells only incubated with 10 μM L for 30 min acted as a control. Confocal luminescence images of HBL-100 cells were carried out using an Olympus FV1000 laser scanning confocal microscope and a 100× oil-immersion objective lens. Emissions were collected at 530–630 nm for the HBL-100 cells.Results and discussion
Synthesis of L
The general synthetic procedure is shown in Scheme 2. The target compound L was easily synthesized via two steps from readily available rhodamine 6G with an overall yield of 58%. The structures of intermediate and L were all confirmed by ESI-mass spectrometry (Fig. S5 and S6†), 1H NMR (Fig. S7 and S9†), 13C NMR (Fig. S8–S10†), 1H–1H Cosy (Fig. S11†), and 13C–1H Cosy (Fig. S12†).UV-vis and fluorescence spectra responses
Absorption and fluorescence spectra were performed in water. As shown in Fig. 1, the free probe L was initially colourless and had almost no absorption above 500 nm in the UV-vis spectrum, which was ascribed to the spirolactam form of L. Upon the addition of the Fe3+ ions (0–4.5 eq.), a significant new absorption band centred at 540 nm was observed, resulting in a colour change from colorless to pink. This suggested the opening of the closed rhodamine–spirolactam ring. Such a significant colour change ensured L as a sensitive “off–on” and colorimetric probe for Fe3+. Moreover, from the absorption titrations, the association constant of L with Fe3+ was calculated to be 3.5 × 104 M−1 according to the Benesi–Hildebrand equation (Fig. 2). | ||
| Fig. 1 The UV-vis absorption titration spectra of L (10 μM) with Fe3+ (0–4.5 equiv.) in water. Inset: digital images of L and L in the presence of an equivalent amount of Fe3+ under normal light. | ||
Probe L showed almost no fluorescence emission upon excitation at 500 nm. With increasing concentrations of Fe3+ ions, the fluorescence titration curve showed a steady and smooth enhancement (Fig. 3). The emission intensity reached its maximum, and the intensity increased about 70-fold after the addition of a 4.5 equivalent of Fe3+. Plotting the fluorescence intensity versus Fe3+ concentration (1–4.5 equiv.) afforded a good linear relationship (R = 0.9984). The detection limit of Fe3+ was calculated from the equation DL = 3S/ρ, where S is the standard deviation of the blank measurement and ρ is the slope between intensity versus sample concentration. The detection limit of L for Fe3+ was 1.9 × 10−8 M (Fig. S1†). The quantum yield for L-Fe3+ was detected to be Φ = 0.78 (L with 10 equiv. Fe3+). The fluorescence intensity became the strongest when the molar fraction of Fe3+ was 0.5, which indicated that the bind stoichiometry between probe L and Fe3+ is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 4).
1 (Fig. 4).
 | ||
| Fig. 3 Fluorescence titrations of L (10 μM) with Fe3+ in distilled water. Inset: fluorescence emission intensity changes with increasing Fe3+ (λex = 500 nm, λem = 560 nm). | ||
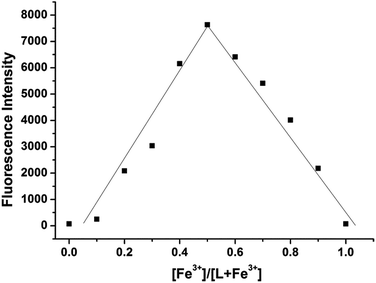 | ||
| Fig. 4 Job's plot of the L-Fe3+ complexes in distilled water keeping the total concentrations of L and Fe3+ at 10 μM. Emission wavelength was 560 nm. | ||
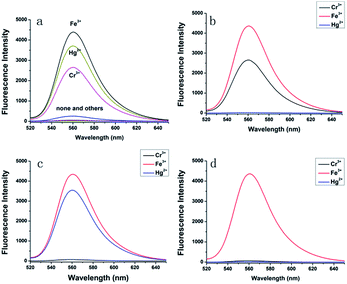
Ionic selectivity
For an excellent probe, high selectivity is an important parameter. We examined the fluorescence spectra of probe L response to various metal ions, including Na+, K+, Li+, Ca2+, Mg2+, Mn2+, Cu2+, Zn2+, Cr3+, Hg2+, Ag+, Cd2+, Ni2+, Co2+, Pb2+, Fe2+ and Fe3+. As shown in Fig. 5a, the sensor itself was almost non-fluorescent due to its ring-closed spirolactam structure. After the addition of Fe3+, a strong emission band centred at 560 nm was observed with an extreme fluorescence enhancement compared to the metal-free L. Interestingly, the addition of Hg2+ and Cr3+ also provided a new peak at 560 nm in the fluorescent spectra with a 60-fold and 45-fold enhancement, which was lower compared with that for Fe3+ with a 70-fold enhancement. This indicated that L had a relatively higher binding affinity to Fe3+ than to Hg2+ and Cr3+. We knew that Cl− and Hg2+ could generate HgCl2 and [HgCl4]2−, whereas triethanolamine (TEOA) and Cr3+ could generate a more stable chelate. We used sodium chloride (NaCl) and TEOA to eliminate the influence of Hg2+ and Cr3+ on the probe, respectively (Fig. 5b and c). When all are added, neither Hg2+ nor Cr3+ will have any effect on the probe with Fe3+ hardly affected (Fig. 5d). TEOA can only eliminate the influence of Cr3+ on the probe and does not affect the determination of Fe3+, when the concentration of TEOA ranged from 50 μM to 100 μM (Fig. S2†). In addition, we also studied the fluorescence spectra of the probe L response to all lanthanide ions except for Pm (radioactive elements). The results indicated that the addition of lanthanide ions did not show any significant enhancement at 560 nm (Fig. S3†).Competition experiment
Competition experiment was conducted with 5 equivalents of Fe3+ ion in the presence of other metal ions in the same concentration, which is demonstrated in Fig. 6. We can observe that most of the detection systems exhibited minimum interference in the detection of Fe3+, Hg2+ and Cr3+ and did not induce any evident interference in the fluorescence sensing of Fe3+. The phenomenon that even 50 equiv. of K+, Na+, Ca2+ could not influence the fluorescence intensity indicates that the probe L may perform well in living cell, where the ions K+, Na+ and Ca2+ are active.Effect of pH and reversibility of L
The effect of pH on fluorescence response of the probe L to Fe3+ was also investigated in water. Without the addition of Fe3+, the ring opening of the probe L occurred under acidic condition (pH < 5), whereas no fluorescence change was observed with pH values of more than 5 (Fig. 7). Upon the addition of 50 μM Fe3+ under identical conditions, fluorescence intensity of probe L had no change in 3–8. In the range of 8–10, fluorescence intensity of L-Fe3+ was sharply decreased with the increase of pH because Fe3+ existed in the form of iron hydroxide under alkaline conditions. It is evident that the wide pH range of 5–8 makes possible the application of probe L in the physiological pH window. This is important for applications in environmental monitoring and living cells. | ||
| Fig. 7 Fluorescence intensity of L (10 μM) at various pH values in distilled water in the absence and presence of Fe3+ (5 equiv.). | ||
Reversibility of the probe is an important phenomenon in developing novel probes for practical applications. In order to demonstrate this reversibility, the emission titration experiment was conducted using the L-Fe3+ complex with Na4P2O7. As shown in Fig. 8, the fluorescence intensity of L-Fe3+ complex was gradually decreased with the increasing concentration of Na4P2O7. These results indicated the decomplexation of L-Fe3+ as Na4P2O7 strips away Fe3+ from the binding zone. When ethanedioic acid, EDTA, tartaric acid and propanedioic acid were introduced into the L-Fe3+ complex, we determine that the fluorescence intensity of the L-Fe3+ complex slightly decreased at 100 eq. EDTA, whereas the remainder exhibit almost no influence to the fluorescence intensity of the L-Fe3+ complex even at 100 eq. ethanedioic acid, tartaric acid and propanedioic acid (Fig. S3†).
 | ||
| Fig. 8 Fluorescence titration spectra of L-Fe3+ (10 μM) upon increasing the concentration of Na4P2O7 (0–10 equivalents). | ||
Fluorescence imaging
Probe L was used for the fluorescence imaging of Fe3+ in living cells because it can detect Fe3+ in water and at physiological pH conditions. HBL-100 cells that incubated with L (10 μM) for 30 min at 37 °C exhibited almost no fluorescence (Fig. 9b). Bright-field measurements after treatment with L confirmed that the cells were viable throughout the imaging experiments. In contrast, the cells stained with both probe L and incubated with Fe3+ (100 μM) displayed bright fluorescence (Fig. 9e). Overlays of confocal fluorescence and bright-field images demonstrated that fluorescence was evident. Therefore, these results indicated that probe L is a cell membrane-permeable, an effective intracellular imaging agent for Fe3+ ions and valuable for studying the uptake, bioaccumulation, and bioavailability of Fe3+ in living organisms.Conclusions
In summary, a novel fluorescence probe L was designed and synthesized. This probe can detect Fe3+ with a fluorescence turn-on effect in water, and the detection limit is 1.9 × 10−8 M. Moreover, the fact that Na4P2O7 could quench the fluorescence of the L-Fe3+ complex indicates the reversibility of probe L. A comparative mild pH range (5–8) provided a possibility for fluorescence imaging experiments. The confocal fluorescence imaging indicated that L is cell permeable and can be used for monitoring intracellular Fe3+ in living cells.Acknowledgements
The financial support from the National Natural Scientific Foundation of China (NNSFC) Project (21431002).Notes and references
- K. P. Carter, A. M. Young and A. E. Palmer, Chem. Rev., 2014, 114, 4564–4601 CrossRef CAS PubMed.
- X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910–1956 CrossRef CAS PubMed.
- J. Du, M. Hu, J. Fan and X. Peng, Chem. Soc. Rev., 2012, 41, 4511–4535 RSC.
- G. Papanikolaou and K. Pantopoulos, Toxicol. Appl. Pharmacol., 2005, 202, 199–211 CrossRef CAS PubMed.
- T. A. Rouault and W. H. Tong, Trends Genet., 2008, 24, 398–407 CrossRef CAS PubMed.
- M. Andjelkovic, J. Vancamp, B. Demeulenaer, G. Depaemelaere, C. Socaciu, M. Verloo and R. Verhe, Food Chem., 2006, 98, 23–31 CrossRef CAS.
- T. Ganz, Blood, 2003, 102, 783–788 CrossRef CAS PubMed.
- K. D. Welch, T. Z. Davis and S. D. Aust, Arch. Biochem. Biophys., 2002, 397, 360–369 CrossRef CAS PubMed.
- J. P. Sumner and R. Kopelman, Analyst, 2005, 130, 528–533 RSC.
- R. R. Crichton, D. T. Dexter and R. J. Ward, Coord. Chem. Rev., 2008, 252, 1189–1199 CrossRef CAS.
- A. S. Dornelles, V. A. Garcia, M. N. de Lima, G. Vedana, L. A. Alcalde, M. R. Bogo and N. Schroder, Neurochem. Res., 2010, 35, 564–571 CrossRef CAS PubMed.
- A. Ohashi, H. Ito, C. Kanai, H. Imura and K. Ohashi, Talanta, 2005, 65, 525–530 CrossRef CAS PubMed.
- Z.-Q. Liang, C.-X. Wang, J.-X. Yang, H.-W. Gao, Y.-P. Tian, X.-T. Tao and M.-H. Jiang, New J. Chem., 2007, 31, 906 RSC.
- Z. O. Tesfaldet, J. F. van Staden and R. I. Stefan, Talanta, 2004, 64, 1189–1195 CrossRef CAS PubMed.
- D. M. Gomes, M. A. Segundo, J. L. Lima and A. O. Rangel, Talanta, 2005, 66, 703–711 CrossRef CAS PubMed.
- S. Lunvongsa, M. Oshima and S. Motomizu, Talanta, 2006, 68, 969–973 CrossRef CAS PubMed.
- Z. Yang, M. She, B. Yin, J. Cui, Y. Zhang, W. Sun, J. Li and Z. Shi, J. Org. Chem., 2012, 77, 1143–1147 CrossRef CAS PubMed.
- N. R. Chereddy, S. Thennarasu and A. B. Mandal, Dalton Trans., 2012, 41, 11753–11759 RSC.
- W. Lin, L. Yuan, J. Feng and X. Cao, Eur. J. Org. Chem., 2008, 2689–2692 CrossRef CAS.
- R. K. Jackson, Y. Shi, X. Yao and S. C. Burdette, Dalton Trans., 2010, 39, 4155–4161 RSC.
- D. P. Kennedy, C. D. Incarvito and S. C. Burdette, Inorg. Chem., 2010, 49, 916–923 CrossRef CAS PubMed.
- B. Sui, S. Tang, T. Liu, B. Kim and K. D. Belfield, ACS Appl. Mater. Interfaces, 2014, 6, 18408–18412 CAS.
- J. Nandre, S. Patil, V. Patil, F. Yu, L. Chen, S. Sahoo, T. Prior, C. Redshaw, P. Mahulikar and U. Patil, Biosens. Bioelectron., 2014, 61, 612–617 CrossRef CAS PubMed.
- S. Li, Y. Li, J. Cao, J. Zhu, L. Fan and X. Li, Anal. Chem., 2014, 86, 10201–10207 CrossRef CAS PubMed.
- M. Zheng, H. Tan, Z. Xie, L. Zhang, X. Jing and Z. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 1078–1083 CAS.
- X. Qu, Q. Liu, X. Ji, H. Chen, Z. Zhou and Z. Shen, Chem. Commun., 2012, 48, 4600–4602 RSC.
- C. Wolf, X. Mei and H. K. Rokadia, Tetrahedron Lett., 2004, 45, 7867–7871 CrossRef CAS.
- J. Huang, Y. Xu and X. Qian, Dalton Trans., 2014, 43, 5983–5989 RSC.
- S. Ji, X. Meng, W. Ye, Y. Feng, H. Sheng, Y. Cai, J. Liu, X. Zhu and Q. Guo, Dalton Trans., 2014, 43, 1583–1588 RSC.
- M. H. Lee, T. V. Giap, S. H. Kim, Y. H. Lee, C. Kang and J. S. Kim, Chem. Commun., 2010, 46, 1407–1409 RSC.
- R. Kagit, M. Yildirim, O. Ozay, S. Yesilot and H. Ozay, Inorg. Chem., 2014, 53, 2144–2151 CrossRef CAS PubMed.
- K. Wu, H. Xiao, L. Wang, G. Yin, Y. Quan and R. Wang, RSC Adv., 2014, 4, 39984 RSC.
- H. Sheng, X. Meng, W. Ye, Y. Feng, H. Sheng, X. Wang and Q. Guo, Sens. Actuators, B, 2014, 195, 534–539 CrossRef CAS.
- S.-R. Liu and S.-P. Wu, Sens. Actuators, B, 2012, 171–172, 1110–1116 CrossRef CAS.
- Z. Ma, F. Yang, Z. Wang and X. Jia, Tetrahedron Lett., 2015, 56, 393–396 CrossRef CAS.
- M. Rajasekar and T. Mohan Das, RSC Adv., 2014, 4, 30976 RSC.
- S. Ding and N. Jiao, Angew. Chem., 2012, 51, 9226–9237 CrossRef CAS PubMed.
- M. Zhang, S. Zhang, G. Zhang, F. Chen and J. Cheng, Tetrahedron Lett., 2011, 52, 2480–2483 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detection limit determination, fluorescence spectra of Fe3+, Cr3+ add in L with different TEOA concentrations, the recognition of the probe for lanthanide ions, the influence of ethanedioic acid, EDTA, tartaric acid and propanedioic acid to the reversibility of the probe, the spectra (1H-NMR, 13C-NMR, ESI-MS) of 1 and L, the spectra of 1H–1H Cosy, 13C–1H Cosy of L. See DOI: 10.1039/c5ra09758d |
| This journal is © The Royal Society of Chemistry 2016 |