Silver(I)-catalysed domino alkyne-annulation/Diels–Alder reaction: a mild synthetic approach to tetrahydrospiro[carbazole-4,3′-indoline] scaffolds†
Pankaj
Sharma‡
a,
Niggula Praveen
Kumar‡
a,
Namballa Hari
Krishna
a,
Daasi
Prasanna
a,
Balasubramanian
Sridhar
b and
Nagula
Shankaraiah
*a
aDepartment of Medicinal Chemistry, National Institute of Pharmaceutical Education and Research (NIPER), Hyderabad-500037, India. E-mail: shankar@niperhyd.ac.in; shankarnbs@gmail.com
bLaboratory of X-ray Crystallography, CSIR-Indian Institute of Chemical Technology, Hyderabad-500007, India
First published on 5th September 2016
Abstract
A mild and efficient AgOTf catalyzed alkyne-annulation/Diels–Alder cascade for the construction of tetrahydrospiro[carbazole-4,3′-indoline] derivatives has been established from N-tosyl-2-(but-3-en-1-yn-1-yl) aniline in the presence of methyleneindolinones. This strategy provides access to the one-pot synthesis of a new class of various novel and structurally complex spirooxindole molecules with high diastereoselectivity.
Spirooxindoles represent an abundant core of privileged heterocyclic molecules which are known to be present in a number of natural products1 (Fig. 1) that exhibit a diverse range of biological properties such as anticancer,1h–l antimalarial1metc. On the other hand, a tetrahydrocarbazole nucleus is also found in a diverse array of naturally occurring alkaloids as well as in pharmacologically active agents.2 For example, (R)-ramatroban is used for coronary artery disease2d and WAY-253752 is utilized as a dual-acting SSRI/5HT1A antagonist.2e Hence, the development of novel and efficient methods for the synthesis of this class of heterocyclic molecules is of great importance as far as synthetic and medicinal aspects are concerned.
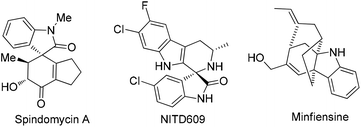 | ||
| Fig. 1 Pharmaceutically important natural products/derivatives containing a spirooxindole and tetrahydrocarbazole core. | ||
During the past few decades, transition metal-catalyzed cyclisation,3 Diels–Alder (DA) cyclizations,4 allenylanilines cyclisation5 and enantioselective hydroarylation of allenes with indoles6 have been intensively explored for the synthesis of tetrahydrocarbazoles. Later, organocatalytic asymmetric versions of DA reactions using enamine,7,8 bifunctional acid base catalysis9 and hydrogen-bonding catalysis10 have also been well reported in the literature. Bernardi and co-workers10a reported a catalytic asymmetric DA reaction of 3-vinylindoles with different dienophiles, whereas, Barbas and co-workers10d presented an elegant approach to the carbazolespirooxindole skeleton from 3-vinylindoles and methyleneindolinones. However, both the reactions were catalyzed by a C2-symmetric bis-thiourea organocatalyst in the cycloaddition reaction.
Next, Melchiorre and co-workers11 developed an asymmetric amino catalyzed DA reaction of ortho-quinodimethanes (oQDMs) which were generated in situ from β-indolyl unsaturated aldehydes with methyleneindolinones for the construction of the spiro[tetrahydrocarbazole-3,3′-oxindole] framework. Recently, Shi et al.12 reported a chiral phosphoric acid catalyzed stereoselective construction of a spiro[tetrahydrocarbazole-3,3′-oxindole] architecture from 2-vinylindoles with methyleneindolinones. Considering the medicinal significance of carbazolespirooxindole and limitations in establishing the challenging spiro-quaternary centre from previous methods, the development of catalytic methods for the synthesis of this class of spirooxindole either by transition metal catalysis or asymmetric organocatalysis is of prime synthetic value.
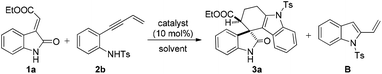
On the other hand, domino reactions13 wherein, at least two or more steps are pooled into one synthetic operation, have attracted considerable attention for the synthesis of complex scaffolds due to their step economy. Intramolecular alkyne-annulations provide access to various substituted indoles from o-alkynylanilines by Lewis acid catalysis.14 Inspired by the pioneering work of annulations on o-alkynylanilines and our earlier efforts in the design and synthesis of medicinally important oxindoles,1i–l,15 we then envisioned a domino reaction between N-tosyl-2-(but-3-en-1-yn-1-yl) aniline and methyleneindolinones for the synthesis of spiro[tetrahydrocarbazole-3,3′-oxindole] scaffolds by employing suitable Lewis acids. Thus, we sought an in situ generation of 1-tosyl-2-vinyl-1H-indole from N-tosyl-2-(but-3-en-1-yn-1-yl) aniline, which then concomitantly reacts with different methyleneindolinones via the [4 + 2] cycloaddition reaction that allows a rapid and direct access to structurally complex tetrahydrospiro[carbazole-4,3′-indoline] in a one pot manner. Moreover, to the best of our knowledge these scaffolds are new and have not yet been reported in the literature (Scheme 1).
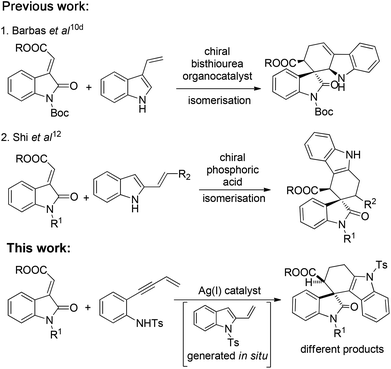 | ||
| Scheme 1 Synthetic strategy comparison with previous approaches to construct spirooxindoles from methyleneindolinones. | ||
In order to explore a suitable Lewis acid to probe the feasibility of this reaction, methyleneindolinone 1a was reacted with N-tosyl-2-(but-3-en-1-yn-1-yl) aniline (2b) under different reaction conditions (Table 1). The frequently used Lewis acids were not effective for this transformation (entries 2–8, Table 1). Usage of copper triflate and silver acetate resulted in aza annulation to form the intermediate 1-tosyl-2-vinyl-1H-indole (entries 4 and 9, Table 1, which has been isolated and characterized by spectroscopic studies) but did not show any influence on the cycloaddition reaction. Among different transition metal catalysts, it was noted that only silver based Lewis acids were effective for this transformation. However, among the screened silver catalysts, AgOAc and AgNO3 were not effective in catalysing this reaction (entries 9 and 10, Table 1). Delightfully, usage of AgSbF6 (10 mol%) resulted in 71% yield of the product 3a in DCE solvent with >99 dr (entry 11, Table 1). Switching the catalyst to AgOTf (10 mol%) delivered the product 3a, albeit with a little improvement in the yield (73%) without changing dr (entry 12, Table 1). Further investigations by screening various gold catalysts such as gold chloride (10 mol%) and triphenylphosphine gold chloride (10 mol%) revealed the fact that these catalysts are ineffective for this transformation (entries 13 and 14, Table 1). Next, we subsequently moved to examine the effect of the solvents on the reaction outcome in terms of yield (entries 15–17, Table 1). Solvents such as toluene and dichloromethane were shown to decrease the yield without affecting the diastereomeric ratio of the product (entries 15 and 16, Table 1), whereas in THF, the reaction did not result in the formation of the required product (entry 17, Table 1). Increasing the reaction temperature to 60 °C resulted in a decrease in the yield (entry 18, Table 1). Decreasing the load of the catalyst from 10 mol% to 5 mol% led to the decrease in the yield (entry 19, Table 1). Moreover, no improvement in the yield was observed on increasing the catalyst load from 10 mol% to 20 mol% (entry 20, Table 1).
| Entry | Catalyst (10 mol%) | Solvent | drb | Yieldc |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.230 mmol), 2b (0.299 mmol), Lewis acid, solvent (DCE, 5 mL), 72 h. b Determined by 1H NMR. c Isolated yields. d 1-Tosyl-2-vinyl-1H-indole was isolated. e Other than 3a, 12% of 1-tosyl-2-vinyl-1H-indole (B) was isolated. f Heating at 60 °C for 12 h. g 5 mol% catalyst. h 20 mol% catalyst. | ||||
| 1 | — | DCE | — | — |
| 2 | Bi(OTf)3 | DCE | — | — |
| 3 | Yb(OTf)3 | DCE | — | — |
| 4 | Cu(OTf)2 | DCE | — | 28d |
| 5 | Al(OTf)3 | DCE | — | — |
| 6 | Sc(OTf)3 | DCE | — | — |
| 7 | In(OTf)3 | DCE | — | — |
| 8 | InCl3 | DCE | — | — |
| 9 | AgOAc | DCE | — | 42d |
| 10 | AgNO3 | DCE | — | — |
| 11 | AgSbF6 | DCE | >99 | 71 |
| 12 | AgOTf | DCE | >99 | 73 |
| 13 | AuCl3 | DCE | — | — |
| 14 | Ph3PAuCl | DCE | — | — |
| 15 | AgOTf | toluene | >99 | 54 |
| 16 | AgOTf | CH2Cl2 | >99 | 51 |
| 17 | AgOTf | THF | — | — |
| 18 | AgOTf | DCE | >99 | 65f |
| 19 | AgOTf | DCE | >99 | 62g |
| 20 | AgOTf | DCE | >99 | 74h |
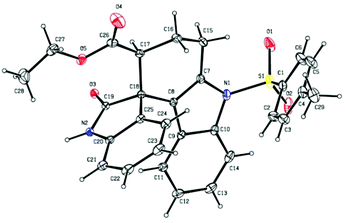
It was quite interesting to observe that the product obtained from the present domino strategy was different from that of the earlier report.121H NMR studies revealed that the characteristic peaks of earlier reported products12 were not present in the obtained product 3a (singlet proton of CH– attached to ester functionality). The regiochemistry of the obtained spiro product was further confirmed by 2D-1H–1H DQFCOSY experiments (see the ESI†) and relative stereochemistry was determined by X-ray crystallographic analysis (Fig. 2).
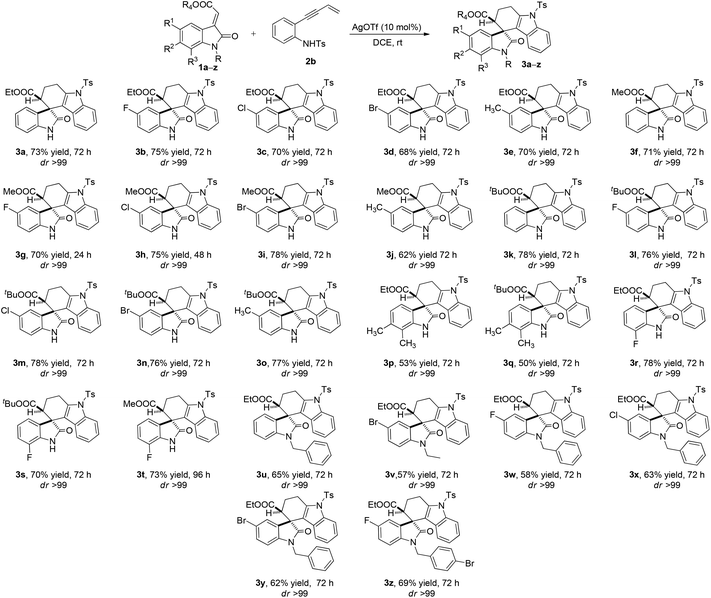
After adopting the optimal reaction conditions the substrate scope of the AgOTf-catalyzed tetrahydrospiro[carbazole-4,3′-indoline] synthesis was examined as shown in Scheme 2. This approach appears to be noteworthy due to the fact that all methyleneindolinones were highly tolerant towards the electronic variation which makes it possible to access a variety of structurally complex tetrahydrospiro[carbazole-4,3′-indoline] derivatives. Methyleneindolinones containing electron-neutral, electron-withdrawing as well as electron-releasing substituents at the C5-position of oxindole were found to be compatible and reacted well under these optimised reaction conditions to access the corresponding products in moderate to good yields (50–78%, 3a–z, Scheme 2) with high diastereoselectivity. Even C7- and C6–C7 disubstituted oxindoles were equally effective for this transformation. Changing the ester part at C-3 alkene of oxindole viz. ethyl (3a–e and 3p, 3r and 3u–zScheme 2), methyl (3f–j and 3tScheme 2) and bulky tertiary butyl (3k–o, 3q and 3sScheme 2) did not show any effect on the reactivity, diastereoselectivity and yields of the reaction as well. Generally, N-substituted oxindoles gave lower yields when compared to the unsubstituted oxindoles without changing the reactivity or diastereoselectivities irrespective of the substitution at C5-, C7- and C6–C7 positions (Scheme 2).
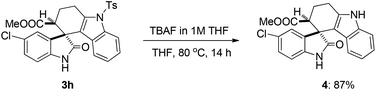
Next, in order to demonstrate the synthetic utility of this method or molecules, one of the representative compounds such as methyl 5′-chloro-2′-oxo-9-tosyl-1,2,3,9-tetrahydrospiro[carbazole-4,3′-indoline]-3-carboxylate (3h) was subjected to detosylation using TBAF to afford the corresponding derivative 4 in 87% yield (Scheme 3).
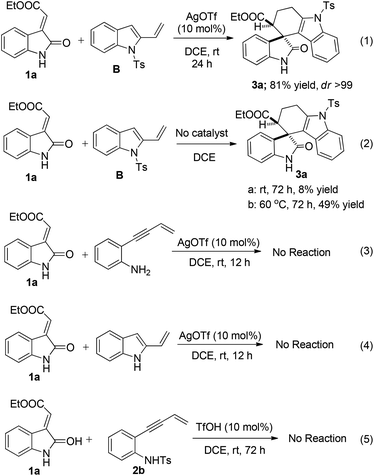
To further elucidate the role of the catalyst and the protecting group in the rate of reaction as well as regioselectivity, few control experiments were performed on N-tosylated, N-detosylated-2-vinyl-1H-indole and 2-(but-3-en-1-yn-1-yl)aniline (Scheme 4). Treatment of 1a with isolated 1-tosyl-2-vinyl-1H-indole (B) in the presence of the catalyst resulted the required product 3a in a comparably shorter duration of the reaction time than the domino process (eqn (1), Scheme 4). However, the reaction was very sluggish in the absence of the catalyst and yielded the same product 3a in less than 10% at room temperature even after stirring the reaction for more than 72 h (eqn (2), Scheme 4), however increasing the temperature to 60 °C improved the yield up to 50% with the formation of unknown by-products. This concludes that AgOTf plays a crucial role in decreasing the activation energy which is required for the cycloaddition process without affecting the regioselectivity of the Diels–Alder reaction. Also to explore the role of the protecting group in regioselectivity, 2-(but-3-en-1-yn-1-yl)aniline (eqn (3), Scheme 4) and N-unsubstituted 2-vinyl-1H-indole (eqn (4), Scheme 4) were treated with 1a which did not yield the required product. This lack of reactivity may be due to stabilization of the Lewis acid by the free amine present in aniline. This confirms that N-protection of 2-vinyl-1H-indole is necessary for this domino process. Further, a control experiment with 1a and 2b in the presence of TfOH (10 mol%) was performed and did not yield the required product 3a (eqn (5), Scheme 4). This experiment suggested that AgOTf is playing an important role in aza-annulation whereas TfOH is unable to catalyze this reaction which excludes the possibility of acid catalysis arising from AgOTf.16
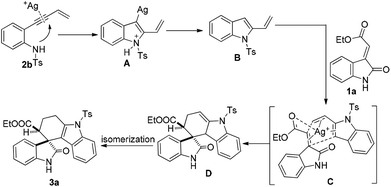
Based on the above observation through control experiments, the possible mechanism for this transformation has been depicted in Scheme 5. Initially, intramolecular aza annulation of 2-ene-yne aniline (2b) would lead to the generation of an isolable 2-vinyl indole Bvia protodemetallation of a transient intermediate A, which then in the presence of methyleneindolinone (1a) would undergo a [4 + 2] cycloaddition to yield tetrahydrospiro[carbazole-4,3′-indoline]. Presumably, it was considered that the silver(I)-catalyst coordinates with ester dienophile17 and makes it more electron deficient, which may lower the energy required for cycloaddition (C) resulting in D which upon isomerisation generates the final product 3a.
In conclusion, an efficient and novel silver(I)-catalyzed domino strategy for the construction of tetrahydrospiro[carbazole-4,3′-indoline] derivatives has been developed from N-tosyl-2-(but-3-en-1-yn-1-yl) aniline in the presence of methyleneindolinones under milder reaction conditions. The reaction proceeds through an in situ generation of 1-tosyl-2-vinyl-1H-indole with concomitant [4 + 2] cycloaddition with high diastereoselectivities. This protocol enriches the toolbox for the synthesis of a new class of spirooxindole scaffolds which could be further exploited in medicinal chemistry. Furthermore, studies towards the asymmetric version of this reaction and evaluation of medicinal activity of these new molecules are underway in our laboratory.
Acknowledgements
We are thankful to DoP, the Ministry of Chemicals & Fertilizers, Govt. of India, New Delhi, for awarding the NIPER fellowship. Dr N. Shankaraiah gratefully acknowledges SERB, DST, Govt. of India for the research grant (YSS-2015-001709).Notes and references
- (a) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748 CrossRef CAS PubMed; (b) K. Guo, T.-T. Fang, J.-Y. Wang, A.-A. Wu, Y.-Z. Wang, J. Jiang, X.-R. Wu, S.-Y. Song, W.-J. Su, Q.-Y. Xu and X.-M. Deng, Bioorg. Med. Chem. Lett., 2014, 24, 4995 CrossRef CAS PubMed; (c) H.-R. Wu, L. Cheng, D.-L. Kong, H.-Y. Huang, C.-L. Gu, L. Liu, D. Wang and C.-J. Li, Org. Lett., 2016, 18, 1382 CrossRef CAS PubMed; (d) R. Dalpozzo, G. Bartoli and G. Bencivenni, Chem. Soc. Rev., 2012, 41, 7247 RSC; (e) J. Wang, E. A. Crane and K. A. Scheidt, Org. Lett., 2011, 13, 3086 CrossRef CAS PubMed; (f) X. Lian, S. Guo, G. Wang, L. Lin, X. Liu and X. Feng, J. Org. Chem., 2014, 79, 7703 CrossRef CAS PubMed; (g) J. J. Badillo, G. E. Arevalo, J. C. Fettinger and A. K. Franz, Org. Lett., 2011, 13, 418 CrossRef CAS PubMed; (h) B. Yu, D.-Q. Yu and H.-M. Liu, Eur. J. Med. Chem., 2015, 97, 673 CrossRef CAS PubMed; (i) K. R. Senwar, P. Sharma, T. S. Reddy, M. K. Jeengar, V. L. Nayak, V. G. M. Naidu, A. Kamal and N. Shankaraiah, Eur. J. Med. Chem., 2015, 102, 413 CrossRef CAS PubMed; (j) P. Sharma, K. R. Senwar, M. K. Jeengar, T. S. Reddy, V. G. M. Naidu, A. Kamal and N. Shankaraiah, Eur. J. Med. Chem., 2015, 104, 11 CrossRef CAS PubMed; (k) K. R. Senwar, T. S. Reddy, D. Thummuri, P. Sharma, V. G. M. Naidu, G. Srinivasulu and N. Shankaraiah, Eur. J. Med. Chem., 2016, 118, 34 CrossRef CAS PubMed; (l) P. Sharma, D. Thummuri, T. S. Reddy, K. R. Senwar, V. G. M. Naidu, G. Srinivasulu, S. K. Bharghava and N. Shankaraiah, Eur. J. Med. Chem., 2016, 122, 584 CrossRef CAS PubMed; (m) M. Rottmann, C. McNamara, B. K. S. Yeung, M. C. S. Lee, B. Zou, B. Russell, P. Seitz, D. M. Plouffe, N. V. Dharia, J. Tan, S. B. Cohen, K. R. Spencer, G. E. González-Páez, S. B. Lakshminarayana, A. Goh, R. Suwanarusk, T. Jegla, E. K. Schmitt, H.-P. Beck, R. Brun, F. Nosten, L. Renia, V. Dartois, T. H. Keller, D. A. Fidock, E. A. Winzeler and T. T. Diagana, Science, 2010, 329, 1175 CrossRef CAS PubMed.
- (a) Y. Feng, M. M. Majireck and S. M. Weinreb, J. Org. Chem., 2014, 79, 7 CrossRef CAS PubMed; (b) J. E. Saxton, Nat. Prod. Rep., 1997, 9, 559 RSC; (c) S. Neogi, A. Roy and D. Naskar, J. Comb. Chem., 2010, 12, 617 CrossRef CAS PubMed; (d) E. Busto, V. Gotor-Fernandez and V. Gotor, J. Org. Chem., 2012, 77, 4842 CrossRef CAS PubMed; (e) A. Nikitenko, D. Evrard, A. L. Sabb, R. L. Vogel, G. Stack, M. Young, M. Lin, B. L. Harrison and J. R. Potoski, Org. Process Res. Dev., 2008, 12, 76 CrossRef CAS; (f) S. Nekkanti, N. P. Kumar, P. Sharma, A. Kamal, F. M. Nachtigall, O. Forero-Doria, L. S. Santos and N. Shankaraiah, RSC Adv., 2016, 6, 2671 RSC.
- For selected examples for transition metal-mediated synthesis of tetrahydrocarbazoles: (a) C. Liu and R. A. Widenhoefer, J. Am. Chem. Soc., 2004, 126, 10250 CrossRef CAS PubMed; (b) C. Liu and R. A. Widenhoefer, Chem. – Eur. J., 2006, 12, 2371 CrossRef CAS PubMed; (c) C. Liu, X. Han, X. Wang and R. A. Widenhoefer, J. Am. Chem. Soc., 2004, 126, 3700 CrossRef CAS PubMed; (d) M. Nazare, C. Schneider, A. Lindenschmidt and D. W. Will, Angew. Chem., Int. Ed., 2004, 43, 4526 CrossRef CAS PubMed; (e) F. Zhao, N. Li, Y.-F. Zhu and Z.-Y. Han, Org. Lett., 2016, 18, 1506 CrossRef CAS PubMed; (f) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9533 CrossRef CAS PubMed; (g) H. Huang and R. Peters, Angew. Chem., Int. Ed., 2009, 48, 604 CrossRef CAS PubMed; (h) V. Pirovano, L. Decataldo, E. Rossi and R. Vicente, Chem. Commun., 2013, 49, 3594 RSC.
- For selected examples of Diels–Alder mediated synthesis of tetrahydrocarbazoles: (a) R. S. Hosmane, S. P. Hiremath and S. W. Schneller, J. Chem. Soc., Perkin Trans. 1, 1973, 2450 RSC; (b) M. Haber and U. Pindur, Tetrahedron, 1991, 47, 1925 CrossRef CAS; (c) W. E. Noland, M. J. Walhstrom, M. J. Konkel, M. E. Brigham, A. G. Trowbridge, L. M. C. Konkel, R. P. Gourneau, C. A. Scholten, N. H. Lee, J. J. Condoluci, T. S. Gac, M. M. Pour and P. M. Radford, J. Heterocycl. Chem., 1993, 30, 81 CrossRef CAS; (d) W. E. Noland, G.-M. Xia, K. R. Gee, M. J. Konkel, M. J. Wahlstrom, J. J. Condoluci and D. L. Rieger, Tetrahedron, 1996, 52, 4555 CrossRef CAS; (e) L. Zhou, B. Xu and J. Zhang, Angew. Chem., Int. Ed., 2015, 127, 9220 CrossRef; (f) C.-B. Chen, X.-F. Wang, Y.-J. Cao, H.-G. Cheng and W.-J. Xiao, J. Org. Chem., 2009, 74, 3532 CrossRef CAS PubMed; (g) R. F. Kester, S. J. Berthel and F. Firooznia, Top. Heterocycl. Chem., 2010, 26, 327 CAS; (h) G. Abbiati, V. Canevari, D. Facoetti and E. Rossi, Eur. J. Org. Chem., 2007, 517 CrossRef CAS; (i) R. P. Pandit and Y. R. Lee, Synthesis, 2012, 2910 CAS; (j) M. Abualnaja, P. G. Waddell, W. Clegg and M. J. Hall, Tetrahedron, 2016, 72, 5798 CrossRef CAS.
- N. Kuroda, Y. Takahashi, K. Yoshinaga and C. Mukai, Org. Lett., 2006, 8, 1843 CrossRef CAS PubMed.
- C. Liu and R. A. Widenhoefer, Org. Lett., 2007, 9, 1935 CrossRef CAS PubMed.
- (a) H. Du and K. Ding, Handbook of Cyclization Reactions, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2010, vol. 1, p. 1 Search PubMed; (b) D. Cheng, Y. Ishihara, B. Tan and C. F. Barbas III, ACS Catal., 2014, 4, 743 CrossRef CAS.
- Selected examples of enamine catalysis: (a) D. B. Ramachary, N. S. Chowdari and C. F. Barbas III, Tetrahedron Lett., 2002, 43, 6743 CrossRef CAS; (b) D. B. Ramachary, K. Anebouselvy, N. S. Chowdari and C. F. Barbas III, J. Org. Chem., 2004, 69, 5838 CrossRef CAS PubMed; (c) S. Samanta, J. Krause, T. Mandal and C. Zhao, Org. Lett., 2007, 9, 2745 CrossRef CAS PubMed; (d) B. Han, Z. He, J. Li, R. Li, K. Jiang, T. Liu and Y. Chen, Angew. Chem., Int. Ed., 2009, 48, 5474 CrossRef CAS PubMed; (e) Z. Jia, H. Jiang, J. Li, B. Gschwend, Q. Li, X. Yin, J. Grouleff, Y. Chen and K. A. Jørgensen, J. Am. Chem. Soc., 2011, 133, 5053 CrossRef CAS PubMed; (f) H. Gotoh and Y. Hayashi, Org. Lett., 2007, 9, 2859 CrossRef CAS PubMed; (g) Y. Hayashi, S. Samanta, H. Gotoh and H. Ishikawa, Angew. Chem., Int. Ed., 2008, 47, 6634 CrossRef CAS PubMed.
- Selected examples of acid–base catalysis: (a) J. Shen, T. T. Nguyen, Y. P. Goh, W. Ye, X. Fu, J. Xu and C. H. Tan, J. Am. Chem. Soc., 2006, 128, 13692 CrossRef CAS PubMed; (b) M. Terada, H. Ube and Y. Yaguchi, J. Am. Chem. Soc., 2006, 128, 1454 CrossRef CAS PubMed; (c) R. P. Singh, K. Bartelson, Y. Wang, H. Su, X. Lu and L. Deng, J. Am. Chem. Soc., 2008, 130, 2422 CrossRef CAS PubMed; (d) Y.-C. Zhang, Q.-N. Zhu, X. Yang, L.-J. Zhou and F. Shi, J. Org. Chem., 2016, 81, 1681 CrossRef CAS PubMed.
- Selected examples of hydrogen-bonding catalysis: (a) C. Gioia, A. Hauville, L. Bernardi, F. Fini and A. Ricci, Angew. Chem., Int. Ed., 2008, 47, 9236 CrossRef CAS PubMed; (b) Y. Huang, A. K. Unni, A. N. Thadani and V. H. Rawal, Nature, 2003, 424, 146 CrossRef CAS PubMed; (c) D. J. Sprague, B. M. Nugent, R. A. Yoder, B. A. Vara and J. N. Johnston, Org. Lett., 2015, 17, 880 CrossRef CAS PubMed; (d) B. Tan, G. Hernandez-Torres and C. F. Barbas III, J. Am. Chem. Soc., 2011, 133, 12354 CrossRef CAS PubMed; (e) L.-J. Huang, J. Weng, S. Wang and G. Lu, Adv. Synth. Catal., 2015, 357, 993 CrossRef CAS.
- Y.-K. Liu, M. N. E. Arceo, S. Vera and P. Melchiorre, J. Am. Chem. Soc., 2011, 133, 15212 CrossRef CAS PubMed.
- Y. Wang, M.-S. Tu, L. Yin, M. Sun and F. Shi, J. Org. Chem., 2015, 80, 3223 CrossRef CAS PubMed.
- (a) J.-C. Wasilke, S. J. Obrey, R. T. Baker and G. C. Bazan, Chem. Rev., 2005, 105, 1001 CrossRef CAS PubMed; (b) K. C. Nicolaou, T. Montagnon and S. A. Snyder, Chem. Commun., 2003, 551 RSC; (c) N. Shankaraiah, N. Markandeya, V. Srinivasulu, K. Sreekanth, Ch. S. Reddy, L. S. Santos and A. Kamal, J. Org. Chem., 2011, 76, 7017 CrossRef CAS PubMed.
- (a) G. Fang and X. Bi, Chem. Soc. Rev., 2015, 44, 8124 RSC; (b) M. Naodovic and H. Yamamoto, Chem. Rev., 2008, 108, 3132 CrossRef CAS PubMed; (c) G. Abbiati and E. Rossi, Beilstein J. Org. Chem., 2014, 10, 481 CrossRef PubMed; (d) M. Alvarez-Corral, M. Munoz-Dorado and I. Rodrıguez-Garcıa, Chem. Rev., 2008, 108, 3174 CrossRef CAS PubMed; (e) J. Liu, X. Xie and Y. Liu, Chem. Commun., 2013, 49, 11794 RSC; (f) N. H. Krishna, A. P. Saraswati, M. Sathish, N. Shankaraiah and A. Kamal, Chem. Commun., 2016, 52, 4581 RSC.
- K. R. Senwar, P. Sharma, S. Nekkanti, M. Sathish, A. Kamal, B. Sridhar and N. Shankaraiah, New J. Chem., 2015, 39, 3973 RSC.
- T. T. Dang, F. Boeck and L. Hintermann, J. Org. Chem., 2011, 76, 9353 CrossRef CAS PubMed.
- S. W. Youn and J. I. Eom, J. Org. Chem., 2006, 71, 6705 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1496246. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00430j |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2016 |