A highly efficient one-pot trifluoromethylation/cyclization reaction of electron-deficient 1,3-conjugated enynes: modular access to trifluoromethylated furans and 2,3-dihydrofurans†
Wei
Zhou
a,
Zhenting
Yue
a and
Junliang
Zhang
*ab
aShanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062, P. R. China. E-mail: jlzhang@chem.ecnu.edu.cn
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Shanghai, 200032, P. R. China
First published on 23rd August 2016
Abstract
A highly efficient one-pot trifluoromethylation/cyclization reaction of conjugated enyne aldehydes and ketones was developed, which provides modular access to highly substituted trifluoromethylated furans and 2,3-dihydrofurans. This method has the advantages of mild reaction conditions, wide functional group tolerance, high yields and being transition metal free.
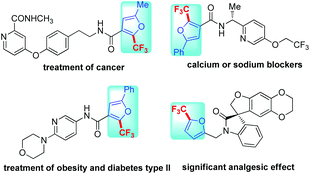
Furans are of significant importance in many fields, including pharmaceuticals, agricultural chemicals and the materials industry.1 In particular, the inclusion of the trifluoromethyl group into these molecules may bring about a dramatic influence on their properties such as bioavailability, metabolic stability and solubility.2 What's more, trifluoromethylated furans are subunits of some biologically active compounds which have been used for treatment of diseases (Fig. 1).3 Consequently, the development of efficient synthetic approaches to trifluoromethylated furans and their derivatives is highly desirable.
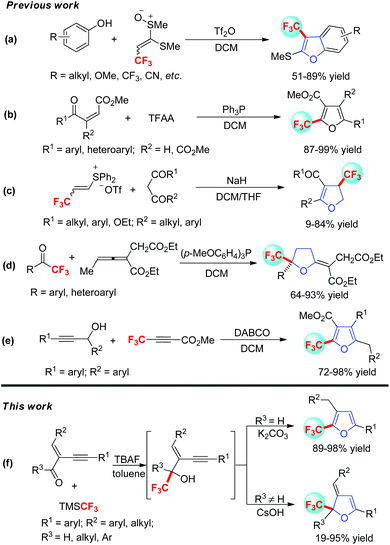
Over the past few decades, numerous synthetic methods have been established for the preparation of trifluoromethylated furans.4 Though direct trifluoromethylation of furans and their derivatives was a step-economy proposal for the construction of these molecules, it's limited by a few drawbacks such as the use of transition metal catalysts, harsh reaction conditions, prohibitive trifluoromethylating regents, poor selectivity and low yields. One of the attractive strategies for the synthesis of trifluoromethyl furan and its derivatives is from the intermolecular reactions of two diverse readily available trifluoromethylated building blocks. For instance, Yorimitsu and co-workers reported a concise and diversity-oriented route to 3-trifluoro-methylbenzo[b]furans from phenols in 2010 (Scheme 1a).5 Later, Xu and co-workers developed a time-economical method for trifluoromethylated furans by using TFAA as the CF3 source (Scheme 1b).6 Subsequently, Lu has developed β-(trifluoromethyl)vinyl sulfonium salts and applied them in the synthesis of trifluoromethylated 2,3-dihydrofurans for the first time (Scheme 1c).7 In 2013, Zhao and co-workers accessed a set of furans containing the CF3-quaternary centre via a phosphine-catalyzed cycloaddition reaction between trifluoromethyl aryl ketone and allenoates (Scheme 1d).8 Recently, Wan and co-workers have developed a highly efficient method for the synthesis of trifluoromethylated furans from propargyl alcohols and methyl 2-perfluoro-alkynoate (Scheme 1e).9 Inspired by these elegant studies and as part of our longstanding interest in tandem cyclization/cycloaddition reactions of conjugated enyne compounds,10,11 we speculated that conjugated enyne ketones or aldehydes might undergo a nucleophilic trifluoromethylation and base promoted cyclization process to generate synthetic valuable trifluoromethylated furans and 2,3-dihydrofurans (Scheme 1f).
Initially, our studies focused on exploring suitable reaction parameters by utilizing conjugated enyne aldehyde 1a as a model substrate (Table 1). Gratifyingly, the nucleophilic trifluoromethylation and K2CO3 promoted aromatic cyclization of 1a proceeded smoothly under mild conditions, delivering the desired trifluoromethylated furan 2a in 76% yield (Table 1, entry 1). Encouraged by this result and for achieving higher yield of 2a, the solvent effect was then investigated (Table 1, entries 2–9). As the results in Table 1 show, toluene proved to be an optimal reaction medium for this reaction and the corresponding 2a was delivered in 91% yield (Table 1, entry 9). Finally, screening of a series of other bases demonstrated that K2CO3 was still the most effective base for this transformation.
| Entry | Solvent | Base | t 1 (h) | t 2 (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Unless otherwise specified, all reactions were carried out with 1a (0.2 mmol), TMSCF3 (0.3 mmol), TBAF (0.3 mmol) in solvent (2 mL) at 0 °C; base (0.4 mmol), 25 °C. b Yield of isolated products. | |||||
| 1 | DCM | K2CO3 | 1 | 14 | 76 |
| 2 | DCE | K2CO3 | 1 | 14 | 69 |
| 3 | CHCl3 | K2CO3 | 1 | 14 | 71 |
| 4 | Et2O | K2CO3 | 1.5 | 14 | 56 |
| 5 | THF | K2CO3 | 1.5 | 14 | 43 |
| 6 | CH3CN | K2CO3 | 1 | 12 | 52 |
| 7 | DMF | K2CO3 | 1 | 12 | 68 |
| 8 | DMSO | K2CO3 | 1 | 12 | 64 |
| 9 | Toluene | K2CO3 | 0.5 | 12 | 91 |
| 10 | Toluene | Na2CO3 | 0.5 | 15 | 86 |
| 11 | Toluene | K3PO4 | 0.5 | 15 | 75 |
| 12 | Toluene | NaOH | 0.5 | 15 | 63 |
| 13 | Toluene | KOH | 0.5 | 15 | 72 |
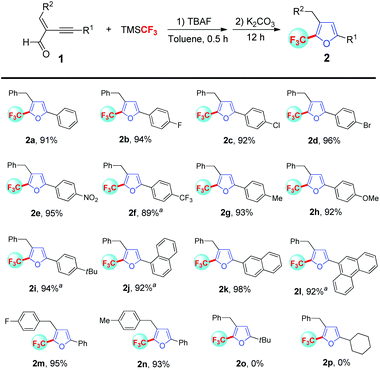
Having identified the optimized reaction conditions, we examined the generality of this process (Scheme 2). Diverse electron-withdrawing groups such as F, Cl, Br, NO2 and CF3 on the phenyl ring of the alkyne moiety were well tolerated and the desired trifluoromethylated furans 2b–2f were delivered in excellent yields. What's more, substrates bearing electron-donating groups such as Me, MeO and tBu on the phenyl ring of the alkyne moiety were also proved to be feasible in this protocol and the corresponding 2g–2i were produced in 92–94% yields. Notably, these trifluoromethylation and cyclization systems were also proved to be efficient for a series of conjugated enyne aldehydes containing a fused ring and the desired 2j–2l were produced in high yields. As illustrated by 1m and 1n, electron-deficient and electron-rich aryl groups on the alkenyl moiety hardly had any effect on this transformation. Unfortunately, conjugated enyne aldehydes 1o and 1p with aliphatic alkyne could give the first addition product but fail to give cycloisomerization product under the present reaction conditions.
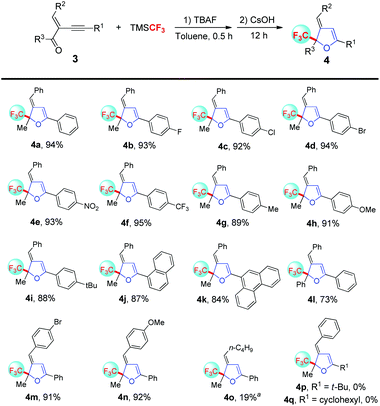
Encouraged by these results, we next concentrated on the trifluoromethylation and cyclization reaction of diverse conjugated enyne ketones (Scheme 3). Gratifyingly, the reaction of conjugated enyne ketone 3a works well, delivering the trifluoromethylated 2,3-dihydrofuran 4a in 94% yield under slightly modified reaction conditions, K2CO3 was replaced by CsOH, the possible reason for this phenomenon might be attributed to the higher nucleophilicity of the corresponding cesium salts of conjugated enyne alcohols due to the larger cationic radius and higher electronic polarizability of Cs+ than Li+, Na+ and K+.12 Additionally, good to excellent yields were generally achieved regardless of the electron-deficient or electron-rich nature of the substituents on the alkyne moiety (4b–4i). What's more, the reaction system was also suitable for conjugated enyne ketones containing the fused ring and the desired 4j–4k were produced in 84–87% yields. More importantly, the phenyl substituted ketone was also compatible, furnishing the desired 4l in moderate yield. Pleasingly, variation of functional groups on the alkenyl moiety was tolerated and the corresponding 4m–4n were generated in good yields. When conjugated enyne ketone 3o (R2 = n-C4H9) was served in the reaction system, a higher reaction temperature (80 °C) was essential for the cyclization process and the desired 4o was obtained in only 19% yield, due to the fact that the unstable 3o easily undergoes isomerization under the reaction conditions. Nevertheless, conjugated enyne ketones 3p and 3q with aliphatic alkyne could give the first addition product but fail cycloisomerization under the present reaction conditions.
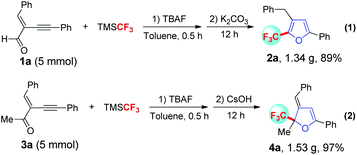
A gram-scale trifluoromethylation/cyclization reaction of conjugated enyne aldehydes and enyne ketones was conducted (Scheme 4). To our delight, 5 mmol of 1a and 3a underwent the trifluoromethylation/cyclization reaction smoothly and gave 1.34 g of 2a and 1.53 g of 4a in 89% and 97% yields, respectively, indicating this method is amendable to the gram-scale.
Conclusions
In summary, we have developed a highly efficient one pot trifluoromethylation/cyclization reaction of conjugated enyne aldehydes and ketones. A variety of trifluoromethylated furans and 2,3-dihydrofurans have been achieved in excellent yields under mild reaction conditions. What's more, gram-scale reactions were also showcased, demonstrating the high efficiency of this strategy. Further studies on the construction of synthetic valuable building blocks from conjugated enyne aldehydes and ketones are underway in our group and will be reported in due course.Acknowledgements
We are grateful to 973 Programs (2015CB856600), the National Natural Science Foundation of China (21372084, 21425205), and the Changjiang Scholars and Innovative Research Team in University (PCSIRT) for financial support.Notes and references
- For selected reports on active furan compounds, see: (a) B. L. Flynn, E. Hamel and M. K. Jung, J. Med. Chem., 2002, 45, 2670 CrossRef CAS PubMed; (b) A. R. Katritzky, S. R. Tala, H. Lu, A. V. Vakulenko, Q.-Y. Chen, J. Sivapackiam, K. Pandya, S. Jiang and A. K. Debnath, J. Med. Chem., 2009, 52, 7631 CrossRef CAS PubMed; (c) L. Racané, V. Tralić-Kulenović, S. K. Pavelić, I. Ratkaj, P. Peixoto, R. Nhili, S. Depauw, M.-P. Hildebrand, M. H. David-Cordonnier, K. Pavelić and G. Karminski-Zamola, J. Med. Chem., 2010, 53, 2418 CrossRef PubMed.
- (a) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef PubMed; (b) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC; (c) O. A. Tomashenko and V. V. Grushin, Chem. Rev., 2011, 111, 4475 CrossRef CAS PubMed; (d) C. Lv, Q. Shen and D. Liu, Chin. J. Org. Chem., 2012, 32, 1380 CrossRef.
- For references on the application of the active trifluoromethylated furan compounds, see: (a) D. Chen, Y. Zhou, Q. Huang and J. Ji, Chin. J. Org. Chem., 1994, 14, 49 CAS; (b) H. Yamamoto, T. Hiyama, K. Kanie, T. Kusumoto, Y. Morizawa and M. Shimzu, Organofluorine Compounds: Chemistry and Application, Springer, Berlin, 2000 Search PubMed; (c) P. Kirsch, Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, Wiley-VCH, Weinheim, Germany, 2004 Search PubMed; (d) D. R. Borcherding, A. Gross, P. W. Shum, N. Willard and B. S. Freed, WO2004100946, 2004 Search PubMed; (e) P. Jeschke, ChemBioChem, 2004, 5, 570 CrossRef CAS PubMed; (f) N. Sakai, S. Imamura, N. Miyamoto and T. Hirayama, WO2008016192, 2008 Search PubMed; (g) K. L. Kirk, Org. Process Res. Dev., 2008, 12, 305 CrossRef CAS.
- For a review see: (a) V. A. Petrov, Fluorinated Heterocyclic Compounds: Synthesis, Chemistry, and Applications, Wiley, Hoboken, NJ, 2009 and references cited therein; for selected examples see: Search PubMed; (b) H. Sawada, M. Nakayama, M. Yoshida, T. Yoshida and N. Kamigata, J. Fluorine Chem., 1990, 46, 423 CrossRef CAS; (c) D. Naumann and J. Kischkewitz, J. Fluorine Chem., 1990, 46, 265 CrossRef CAS; (d) R. Bucci, G. Laguzzi, M. L. Pompili and M. Speranza, J. Am. Chem. Soc., 1991, 113, 4544 CrossRef CAS; (e) R. J. Linderman, E. A. Jamois and S. D. Tennyson, J. Org. Chem., 1994, 59, 957 CrossRef CAS; (f) W. Pang, S. Zhu, Y. Xin, H. Jiang and S. Zhu, Tetrahedron, 2010, 66, 1261 CrossRef CAS; (g) D. Zhang and C. Yuan, Eur. J. Org. Chem., 2007, 3916 CrossRef CAS; (h) Y. Ye and M. S. Sanford, J. Am. Chem. Soc., 2012, 134, 9034 CrossRef CAS PubMed.
- T. Kobatake, D. Fujino, S. Yoshida, H. Yorimitsu and K. Oshima, J. Am. Chem. Soc., 2010, 132, 11838 CrossRef CAS PubMed.
- Y. Wang, Y.-C. Luo, X.-Q. Hu and P.-F. Xu, Org. Lett., 2011, 13, 5346 CrossRef CAS PubMed.
- H. Lin, Q. Shen and L. Lu, J. Org. Chem., 2011, 76, 7359 CrossRef CAS PubMed.
- H. Xiao, Z. Chai, R.-S. Yao and G. Zhao, J. Org. Chem., 2013, 78, 9781 CrossRef CAS PubMed.
- Q. Chong, X. Xin, C. Wang, F. Wu, H. Wang, J.-C. Shi and B. Wan, J. Org. Chem., 2014, 79, 2105 CrossRef CAS PubMed.
- For selected reports related to conjugated enyne compounds by our group see: (a) Y. Xiao and J. Zhang, Angew. Chem., Int. Ed., 2008, 47, 1903 CrossRef CAS PubMed; (b) F. Liu, Y. Yu and J. Zhang, Angew. Chem., Int. Ed., 2009, 48, 5505 CrossRef CAS PubMed; (c) F. Liu, D. Qian, L. Li, X. Zhao and J. Zhang, Angew. Chem., Int. Ed., 2010, 49, 6669 CrossRef CAS PubMed; (d) H. Gao, X. Zhao, Y. Yu and J. Zhang, Chem. – Eur. J., 2010, 16, 456 CrossRef CAS PubMed; (e) H. Gao, X. Wu and J. Zhang, Chem. – Eur. J., 2011, 17, 2838 CrossRef CAS PubMed; (f) H. Gao, X. Wu and J. Zhang, Chem. – Eur. J., 2011, 17, 2838 CrossRef CAS PubMed; (g) X. Yu and J. Zhang, Chem. – Eur. J., 2012, 18, 12945 CrossRef CAS PubMed; (h) H. Gao and J. Zhang, Chem. – Eur. J., 2012, 18, 2777 CrossRef CAS PubMed; (i) H. Qian, X. Yu, J. Zhang and J. Sun, J. Am. Chem. Soc., 2013, 135, 18020 CrossRef CAS PubMed; (j) M. Zhang and J. Zhang, Chem. Commun., 2012, 48, 6399 RSC; (k) X. Yu and J. Zhang, Chem. Commun., 2012, 48, 4002 RSC; (l) Z.-M. Zhang, P. Chen, W. Li, Y. Niu, X. Zhao and J. Zhang, Angew. Chem., Int. Ed., 2014, 53, 4350 CrossRef CAS PubMed; (m) L. Zhou, M. Zhang, W. Li and J. Zhang, Angew. Chem., Int. Ed., 2014, 53, 6542 CrossRef CAS PubMed; (n) W. Li and J. Zhang, Org. Lett., 2014, 16, 162 CrossRef CAS PubMed; (o) M. Zhang and J. Zhang, Chem. – Eur. J., 2014, 20, 399 CrossRef CAS PubMed; (p) Y. Wang, P. Zhang, D. Qian and J. Zhang, Angew. Chem., Int. Ed., 2015, 54, 14849 CrossRef CAS PubMed; (q) W. Li, L. Gao, Z. Yue and J. Zhang, Adv. Synth. Catal., 2015, 357, 2651 CrossRef CAS.
- For selected reports related to conjugated enyne compounds by other groups see: (a) T. Yao, X. Zhang and R. C. Larock, J. Am. Chem. Soc., 2004, 126, 11164 CrossRef CAS PubMed; (b) N. T. Patil, H. Wu and Y. Yamamoto, J. Org. Chem., 2005, 70, 4531 CrossRef CAS PubMed; (c) Y. Liu and S. Zhou, Org. Lett., 2005, 7, 4609 CrossRef CAS PubMed; (d) T. Yao, X. Zhang and R. C. Larock, J. Org. Chem., 2005, 70, 7679 CrossRef CAS PubMed; (e) C. H. Oh, V. R. Reddy, A. Kim and C. Y. Rhim, Tetrahedron Lett., 2006, 47, 5307 CrossRef CAS; (f) C.-H. Cho and R. C. Larock, ACS Comb. Sci., 2011, 13, 272 CrossRef CAS PubMed; (g) Y. Xia, R. Ge, L. Chen, Z. Liu, Q. Xiao, Y. Zhang and J. Wang, J. Org. Chem., 2015, 80, 7856 CrossRef CAS PubMed; (h) F. Hu, Y. Xia, C. Ma, Y. Zhang and J. Wang, J. Org. Chem., 2016, 81, 3275 CrossRef CAS PubMed; (i) Q. Yao, Y. Liao, L. Lin, X. Lin, J. Ji, X. Liu and X. Feng, Angew. Chem., Int. Ed., 2016, 55, 1859 CrossRef CAS PubMed; (j) Y. Xia, L. Chen, P. Qu, G. Ji, S. Feng, Q. Xiao, Y. Zhang and J. Wang, 2016, 81, DOI:10.1021/acs.joc.6b00730.
- (a) C. Galli, Org. Prep. Proced. Int., 1992, 24, 287 CrossRef and references therein; (b) D. Tzalis, C. Koradin and P. Knochel, Tetrahedron Lett., 1999, 40, 6193 CrossRef CAS; (c) R. N. Salvatore, A. S. Nagle, S. E. Schmidt and K. W. Jung, Org. Lett., 1999, 1, 1893 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, analytical data, NMR spectra of products. See DOI: 10.1039/c6qo00385k |
| This journal is © the Partner Organisations 2016 |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) The cyclization reaction was performed at 50 °C for 3 h.
The cyclization reaction was performed at 50 °C for 3 h.