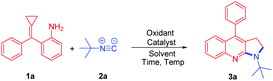An isocyanide insertion approach to substituted pyrrolo[2,3-b]quinolines under metal-free and azide-free conditions†
Cheng-Guo
Liu
,
Zheng-Yang
Gu
,
Hui-Wen
Bai
,
Shun-Yi
Wang
* and
Shun-Jun
Ji
*
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou, 215123, P. R. China. E-mail: shunyi@suda.edu.cn; shunjun@suda.edu.cn; Fax: +86-512-65880307; Tel: +86-512-65880307
First published on 8th August 2016
Abstract
A new strategy for the catalytic synthesis of the pyrrolo[2,3-b]quinoline skeleton by the reaction of isocyanides with (cyclopropylidene(aryl)methyl)aniline (amino-MCPs) is presented, taking advantage of I2/CHP mediated carbodiimide intermediate generation. This reaction involves three C–N bonds and one C–C bond generation under metal-free and azide-free conditions.
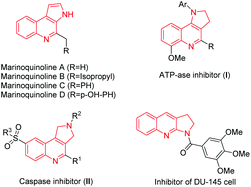
Nitrogen-containing heterocycles, especially pyrrole and quinolone ring systems,1 are a common skeleton unit featured in several natural products and biologically active compounds.2 Furthermore, these compounds are extremely important in the areas of materials science and medicinal chemistry. Some of the examples are illustrated in Scheme 1. Marinoquinolines A–D have shown activity against Plasmodium falciparum K1 with good IC50 values.3 ATPase inhibitor (I) has been shown to work as an energy transfer inhibitor in intact cells of Rhodopseudomonas capsulata.4 Caspase 1 Inhibitor II has neuroprotective function and is able to rescue neuronal cell cultures from cell death.5 Proliferation inhibitor of DU-145 cell was used as a classical cell line of prostate cancer,6 which contained a pyrrole-fused quinoline skeleton. Intensive research has been developed for the synthesis of this kind of pyrrole-fused quinoline moiety.7 However, most of these methods have shortcomings such as the use of explosive and toxic substrates, low reaction scopes and expensive metal catalysts, which limit further applications.8
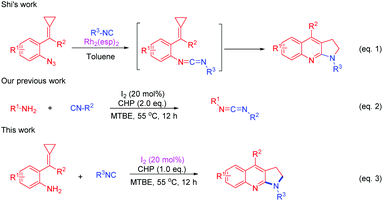
Isocyanides are valuable synthons that are widely used in modern organic synthesis and they played an important role in the construction of nitrogen-containing heterocycles, due to their diversity.9 Methylenecyclopropanes (MCPs) are important building blocks due to their high ring strain and unique electronic properties.10 They are very reactive and can undergo a variety of ring-opening reactions to construct complex molecules.11–14 Shi's group has made an important contribution toward constructing nitrogen heterocycles using MCPs12–14 such as azide-MCPs13 and amino-MCPs.14 Recently, Shi's group reported a Rh(II)-catalyzed intermolecular cyclization from azide-MCPs and isonitriles to afford pyrrolo[2,3-b]quinolones (Scheme 2, eqn (1))13a and fused indoles.13b They have also applied amino-MCPs to construct furoquinoline, thienoquinoline, and benzazepine derivatives.14 Meanwhile, our group has also reported an iodine (I2)/cumene hydroperoxide (CHP) mediated cross-coupling reaction of isocyanides with amines to afford carbodiimides (Scheme 2, eqn (2)).15 Stimulated by Shi's and our own work, herein, we report an I2/CHP mediated reaction of amino-MCPs and isonitriles to synthesize the pyrrolo-fused quinoline skeleton under metal-free and azide-free conditions (Scheme 2, eqn (3)).
 | ||
| Scheme 2 Oxidative reactions of amine with isocyanide and ring expansion and cyclization with isonitriles. | ||
We initiated the studies by reacting 2-(cyclopropylidene(phenyl)methyl)aniline 1a and tert-butyl isonitrile 2a catalyzed by Co(acac)2 (30 mol%) in 1,4-dioxane at 100 °C under an O2 atmosphere (Table 1, entry 1). Unfortunately, the reaction was messy and gave unsatisfactory results. Next, we tried the reaction by utilizing the I2/CHP system. To our delight, the desired product pyrrolo[2,3-b]quinoline 3a was isolated in 71% yield (Table 1, entry 2). When other iodo-source catalysts such as TBAI (tert-butylammonium iodide) and NIS (N-iodosuccinimide) were applied to the reaction, 3a was obtained in poor yields (Table 1, entries 3 and 4). The reaction was further screened in different solvents. Ether solvents, such as MTBE (methyl tert-butyl ether), 1,4-dioxane, and DME (dimethoxyethane), gave the products in moderate yields (Table 1, entries 2, 5 and 9). Other solvents such as DCE, toluene and MeCN decreased the yields to 23%, 35% and 27%, respectively (Table 1, entries 6–8). Further screening of different oxidants, such as CHP, TBPB (tert-butyl peroxybenzoate), TBHP (tert-butyl hydroperoxide), DTBP (2-(tert-butylperoxy)-2-methylpropane), K2S2O8, and O2, showed that CHP was the best oxidant for this type of reaction (Table 1, entries 2 and 10–14). Decreasing the amount of CHP used from 2.0 to 1.0 equiv. improves the formation of 3a to 83% yield (Table 1, entry 17). Therefore, the optimum reaction conditions will be 1a (0.3 mmol, 1.0 equiv.) and 2a (0.36 mmol, 1.2 equiv.) in MTBE (1.5 mL) in the presence of 20% mol of I2 and 1.0 equiv. of CHP as the oxidant at 55 °C for 12 h.
| Entry | Catalyst (mol %) | Oxidant (equiv.) | Solvent | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: Compound 1a (0.2 mmol), tert-butyl isocyanide 2a (1.2 equiv., 0.24 mmol), catalyst, and oxidant in 1.2 mL of solvent at 55 °C in a sealed reaction tube for 12 h. b Yields were determined by HPLC analysis with biphenyl as the internal standard. c Isolated yield. | |||||
| 1 | Co(acac)2(30) | O2 | 1,4-Dioxane | 100 | Mess |
| 2 | I2(20) | CHP(2) | MTBE | 55 | 75(71)c |
| 3 | TBAI (20) | CHP(2) | MTBE | 55 | Trace |
| 4 | NIS (20) | CHP(2) | MTBE | 55 | 18 |
| 5 | I2(20) | CHP(2) | 1,4-Dioxane | 55 | 58 |
| 6 | I2(20) | CHP(2) | DCE | 55 | 23 |
| 7 | I2(20) | CHP(2) | Toluene | 55 | 35 |
| 8 | I2(20) | CHP(2) | MeCN | 55 | 27 |
| 9 | I2(20) | CHP(2) | DME | 55 | 44 |
| 10 | I2(20) | TBPB(2) | MTBE | 55 | Trace |
| 11 | I2(20) | TBHP(2) | MTBE | 55 | 64 |
| 12 | I2(20) | DTBP(2) | MTBE | 55 | 25 |
| 13 | I2(20) | K2S2O8(2) | MTBE | 55 | 23 |
| 14 | I2(20) | O2 | MTBE | 55 | 17 |
| 15 | I2(10) | CHP(2) | MTBE | 55 | 26 |
| 16 | I2(30) | CHP(2) | MTBE | 55 | 39 |
| 17 | I 2 (20) | CHP(1) | MTBE | 55 | 88(83) |
| 18 | I2(20) | CHP(3) | MTBE | 55 | 66 |
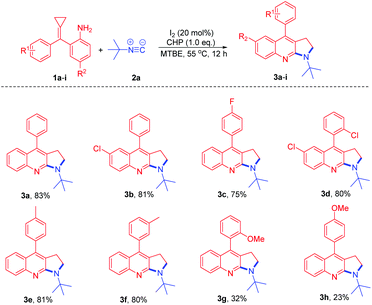
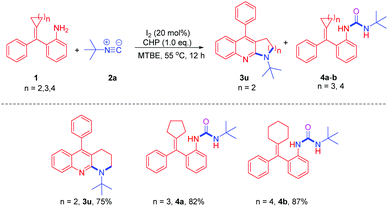
With the optimum reaction conditions in hand, we explored the reaction of t-butyl isocyanide with a variety of amino-MCPs (Scheme 3). Most of the reactions proceeded successfully to afford the desired pyrrolo[2,3-b]quinoline products in moderate to good yields. When halogen-substituted amino-MCPs 1b–d were subjected to the reaction conditions, the corresponding products 3b–d were observed in 75–81% yield. The reaction of amino-MCPs 1e and 1f proceeded smoothly to furnish the pyrrolo[2,3-b]quinoline derivatives 3e and 3f in 80% and 81% yield, respectively (Scheme 3). However, both MCPs 3g and 3h bearing an electron-donating group resulted in a poor yield under identical conditions.
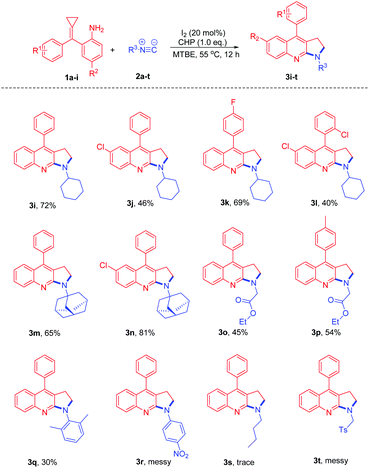
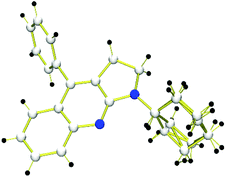
After examining different amino-MCPs, we next investigated the various isocyanides under identical conditions and the results are summarized in Scheme 4. When cyclohexyl isocyanide and adamantyl isocyanide were subjected to the reactions with amino-MCPs, the desired pyrrolo[2,3-b]quinoline derivatives 3i–n can be observed in moderate to good yields (40–81%) (Scheme 4). The structure of 3i was further confirmed by X-ray diffraction (Fig. 1). The reactions of ethyl isocyanatoacetate with 1a and 1e also proceeded smoothly to afford the desired products 3o and 3p in 45% and 54% yield, respectively. It should be noted that 2,6-dimethyl-isocyanide with bulky substituents could also lead to the desired product 3q in 30% yield. Unfortunately, n-butyl isocyanide and 4-nitrophenyl isocyanide with a strong electron withdrawing group failed to proceed under the given reaction conditions.
Since MCPs are highly strained, they can easily undergo a variety of ring-opening rearrangement reactions to afford pyrrolo-fused quinoline compounds. We then further explored the reaction of methylenecyclobutanes 1u with tert-butyl isocyanide 2a under identical conditions. To our delight, the ring-opening rearrangement product 1-(tert-butyl)-5-phenyl-1,2,3,4-tetrahydrobenzo[b][1,8]naphthyridine 3u was observed in 75% yield (Scheme 5).
However, the reactions of 2-(cyclopentylidene(phenyl)methyl)aniline 1a′ and 2-(2-ethyl-1-phenylhex-1-en-1-yl)aniline 1b′ with 2a gave ureas 4a and 4b in 82% and 87% yield (Scheme 5), respectively, instead of the ring-opening rearrangement products. These results indicated that cyclopentane or cyclohexane rings are stable enough, thus it was difficult for them to undergo a thermally-induced ring-opening rearrangement.
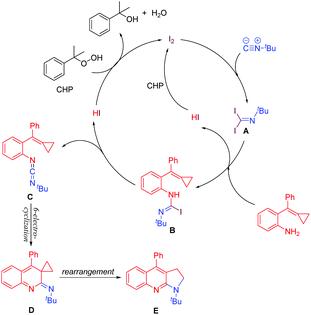
Based on the above experiment results and literature reports,11d,13 we proposed a plausible mechanism as shown in Scheme 6. First, 1,1-addition of iodine to isocyanide gives intermediate A. Intermediate A reacts with amino-MCPs to give intermediate B by dehydrohalogenation. A second dehydrohalogenation subsequently forms the key intermediate, carbodiimide C. Hydrogen iodide is oxidized by CHP to iodine and recycled back to the catalytic cycle. Carbodiimide C is transformed to intermediate D via a consecutive 6p-electrocyclization. After a thermally-induced rearrangement, product E is observed.
Conclusions
In summary, we have developed an I2/CHP catalyzed reaction of amino-MCPs and isonitriles for the synthesis of pyrrolo[2,3-b]quinolones in moderate to good yields. Significantly, this reaction involves a metal-free and azide-free strategy for the construction of structurally novel and useful heterocycles via the carbodiimide intermediate.We gratefully acknowledge the Natural Science Foundation of China (21372174, 21542015), PAPD, the Major Basic Research Project of the Natural Science Foundation of the Jiangsu Higher Education Institutions (no. 16KJA150002), and Soochow University for financial support, and the State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials. We gratefully thank Prof. Min Shi (East China University of Science and Technology, China) for helpful discussion.
Notes and references
- (a) F. Bellina and R. Rossi, Tetrahedron, 2006, 62, 7213 CrossRef CAS; (b) V. Estevez, M. Villacampa and J. C. Menendez, Chem. Soc. Rev., 2010, 39, 4402 RSC; (c) J. P. Michael, Nat. Prod. Rep., 2008, 25, 166 RSC.
- Synthesis of pyrrolo[2,3-b]quinolines: (a) M. A. Khan and J. F. Da Rocha, Heterocycles, 1977, 6, 1229 CrossRef CAS; (b) T. Saito, N. Furukawa and T. Otani, Org. Biomol. Chem., 2010, 8, 1126 RSC; (c) R. Richter and H. Ulrich, J. Org. Chem., 1973, 38, 2614 CrossRef CAS; (d) L. Smith and A. S. Kiselyov, Tetrahedron Lett., 1999, 40, 5643 CrossRef CAS.
- (a) T. H. Brown, R. J. Keeling, C. A. Leach, M. E. Parsons, C. A. D. Price, R. Reavill and K. J. Wiggall, J. Med. Chem., 1990, 33, 527 CrossRef CAS PubMed; (b) C. A. Leach, T. H. Brown, D. J. Laing, S. M. Keeling, M. E. Parsons, C. A. Price and K. J. Wiggall, J. Med. Chem., 1992, 35, 1845 CrossRef CAS PubMed; (c) C. Escolano and K. Jones, Tetrahedron Lett., 2000, 41, 8951 CrossRef CAS.
- (a) A. R. Carroll, S. Duffy and V. M. Avery, J. Org. Chem., 2010, 75, 8291 CrossRef CAS PubMed; (b) Y. Sangnoi, O. Sakulkeo, S. S. Yuenyong, O. A. A. Plubrukarn and K. Suwanborirux, Mar. Drugs, 2008, 6, 578 CrossRef CAS PubMed; (c) K. P. Srisu, C. Suwannachart, O. A. Kanjana, S. Hosoya, A. Yokota and V. Arunpairojana, Int. J. Syst. Evol. Microbiol., 2007, 54, 2275 Search PubMed.
- (a) D. V. Kravchenko, V. M. Kysil, S. E. Tkachenko, S. Maliarchouk, I. M. Okun and A. V. Ivachtchenko, Farmaco, 2005, 60, 804 CrossRef CAS PubMed; (b) D. V. Kravchenko, S. E. Tkachenko, S. Maliarchouk, I. M. Okun and A. V. Ivachtchenko, Eur. J. Med. Chem., 2005, 40, 1377 CrossRef CAS PubMed; (c) D. V. Kravchenko, V. M. Kysil, S. E. Tkachenko, S. Maliarchouk, I. M. Okun and A. V. Ivachtchenko, Farmaco, 2005, 15, 1841 CAS; (d) D. V. Kravchenko, V. M. Kysil, S. E. Tkachenko, S. Maliarchouk and A. V. Ivachtchenko, Lett. Drug Des. Discovery, 2006, 3, 61 CrossRef CAS; (e) D. V. Kravchenko, V. M. Kysil, S. E. Tkachenko, S. Maliarchouk, I. M. Okun and A. V. Ivachtchenko, J. Biomol. Screening, 2006, 11, 694 CrossRef PubMed; (f) S. Sharma, K. Sahu, P. Jain and R. K. Agrawal, Med. Chem. Res., 2008, 17, 399 CrossRef CAS.
- A. A. Gakh, Anti-cancer agents based on N-acyl-2,3-dihydrolHpyrro lo[2,3-b] quinoline derivatives and a method of making, US Pat, 8420815Bl, 2013 Search PubMed.
- Synthesis of pyrrolo[1,2-a]quinolines: (a) E. R. H. Jones, J. Chem. Soc., 1964, 5907 RSC; (b) R. M. Acheson, G. Procter and S. R. Critchley, J. Chem. Soc., 1976, 692 CAS; (c) W. J. Irwin and D. G. Wibberley, J. Chem. Soc., Perkin Trans. 1, 1974, 250–252 RSC; (d) B. C. Uff, R. S. Budhram, M. F. Consterdine, J. K. Hicks, B. P. Slingsby and J. A. Pemblington, J. Chem. Soc., Perkin Trans. 1, 1977, 2018 RSC; (e) S. W. Schneller and J.-K. Luo, J. Org. Chem., 1980, 45, 4045 CrossRef CAS.
- (a) M. Schmittel, J. P. Steffen, B. Engels, C. Lennartz and M. Hanrath, Angew. Chem., Int. Ed., 1998, 37, 2371 CrossRef CAS; (b) X.-L. Lu, L. Jeffrey and K.-K. Petersen, J. Org. Chem., 2002, 67, 5412 CrossRef CAS PubMed; (c) W. Du and D. P. Curran, Org. Lett., 2003, 5, 1765 CrossRef CAS PubMed; (d) M. Schmittel, J. P. Steffen, D. Rodrıguez, B. Engelen, E. Neumann and M. E. Cinar, J. Org. Chem., 2008, 73, 3005 CrossRef CAS PubMed; (e) K. Chen, X.-Y. Tang and M. Shi, Chem. Commun., 2016, 52, 1967 RSC.
- (a) T.-H. Zhu, S.-Y. Wang, G.-N. Wang and S.-J. Ji, Chem. – Eur. J., 2013, 19, 5850 CrossRef CAS PubMed; (b) Y. Li, G. Qiu, Q. Ding and J. Wu, Tetrahedron, 2014, 70, 4652 CrossRef CAS; (c) T. Xiao, L. Li, G. Lin, Q. Wang, P. Zhang, Z.- W. Mao and L. Zhou, Green Chem., 2014, 16, 2418 RSC; (d) J. Liu, G. Zhang, X. Zhang, H. Yi and A. Lei, Chem. Commun., 2014, 50, 2145 RSC.
- For selected reviews, see: (a) M. Lautens, W. Klute and W. Tam, Chem. Rev., 1996, 96, 49 CrossRef CAS PubMed; (b) I. Nakamura and Y. Yamamoto, Adv. Synth. Catal., 2002, 344, 111 CrossRef CAS; (c) A. Brandi, S. Cicchi, F. M. Cordero and A. Goti, Chem. Rev., 2003, 103, 1213 CrossRef CAS PubMed; (d) M. Rubin, M. Rubina and V. Gevorgyan, Chem. Rev., 2007, 107, 3117 CrossRef CAS PubMed; (e) L.-X. Shao and M. Shi, Curr. Org. Chem., 2007, 11, 1135 CrossRef CAS; (f) M. Shi, L.-X. Shao, J.-M. Lu, Y. Wei, K. Mizuno and H. Maeda, Chem. Rev., 2010, 110, 5883 CrossRef CAS PubMed; (g) H. Pellissier, Tetrahedron, 2010, 66, 8341 CrossRef CAS; (h) G. Audran and H. Pellissier, Adv. Synth. Catal., 2010, 352, 575 CrossRef CAS; (i) A. Masarwa and I. Marek, Chem. – Eur. J., 2010, 16, 9712 CrossRef CAS PubMed; (j) M. Shi, J.-M. Lu, Y. Wei and L.-X. Shao, Acc. Chem. Res., 2012, 45, 641 CrossRef CAS PubMed; (k) A. Brandi, S. Cicchi, F. M. Cordero and A. Goti, Chem. Rev., 2014, 114, 7317 CrossRef CAS PubMed; (l) H. Pellissier, Tetrahedron, 2014, 70, 4991 CrossRef CAS.
- (a) M. E. Scott, Y. Bethuel and M. Lautens, J. Am. Chem. Soc., 2007, 129, 1482 CrossRef CAS PubMed; (b) R. J. Felix, D. Weber, O. Gutierrez, D. J. Tantillo and M. R. Gagne, Nat. Chem., 2012, 4, 405 CrossRef CAS PubMed; (c) P. A. Evans and P. A. Inglesby, J. Am. Chem. Soc., 2012, 134, 3635 CrossRef CAS PubMed; (d) P. A. Inglesby, J. Bacsa, D. E. Negru and P. A. Evans, Angew. Chem., Int. Ed., 2014, 53, 3952 CrossRef CAS PubMed; (e) L. Saya, I. Fernandez, F. Lopez and J. L. Mascarenas, Org. Lett., 2014, 16, 5008 CrossRef CAS PubMed; (f) J. Sheng, C. Fan, Y. Ding, X. Fan and J. Wu, Chem. Commun., 2014, 50, 4188 RSC; (g) L. Yu, Y.-L. Wu, T. Chen, Y. Pan and Q. Xu, Org. Lett., 2013, 15, 144 CrossRef CAS PubMed; (h) Q. Xiao, S.-Q. Ye and J. Wu, Org. Lett., 2012, 14, 3430 CrossRef CAS PubMed.
- (a) M. Shi, L.-P. Liu and J. Tang, J. Am. Chem. Soc., 2006, 128, 7430 CrossRef CAS PubMed; (b) K. Chen, M. Jiang, Z. Zhang, Y. Wei and M. Shi, Eur. J. Org. Chem., 2011, 7189 CrossRef CAS; (c) K. Chen, R. Sun, Q. Xu, Y. Wei and M. Shi, Org. Biomol. Chem., 2013, 11, 3949 RSC; (d) R. Sang, X.-Y. Tang and M. Shi, Org. Chem. Front., 2014, 1, 770 RSC; (e) Z.-B. Zhu and M. Shi, Org. Lett., 2010, 12, 4462 CrossRef CAS PubMed; (f) Z.-Z. Zhu, K. Chen, L.-Z. Yu, X.-Y. Tang and M. Shi, Org. Lett., 2015, 17, 5994 CrossRef CAS PubMed; (g) W. Fang, X.-Y. Tang and M. Shi, RSC Adv., 2016, 6, 40474 RSC.
- (a) K. Chen, Z. Zhang, Y. Wei and M. Shi, Chem. Commun., 2016, 52, 1967 RSC; (b) K. Chen, Z.-Z. Zhu, J.-X. Liu, X.-Y. Tang, Y. Wei and M. Shi, Chem. Commun., 2016, 52, 350 RSC.
- (a) K. Chen, Z. Zhang, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 7698 Search PubMed; (b) L.-Z. Yu, X.-B. Hu, Q. Xu and M. Shi, Chem. Commun., 2016, 52, 2701 RSC; (c) L.-Z. Yu, Z.-Z. Zhu, X.-B. Hu, X.-Y. Tang and M. Shi, Chem. Commun., 2016, 52, 6581 RSC; (d) L.-Z. Yu, Q. Xu, X.-Y. Tang and M. Shi, ACS Catal., 2016, 6, 526 CrossRef CAS.
- T.-H. Zhu, S.-Y. Wang, Y.-Q. Tao and S.-J. Ji, Org. Lett., 2015, 17, 1974 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1494641. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00373g |
| This journal is © the Partner Organisations 2016 |