Intramolecular cross dehydrogenative coupling of 4-substituted coumarins: rapid and efficient access to coumestans and indole[3,2-c]coumarins†
Chao
Cheng
ab,
Wen-Wen
Chen
*b,
Bin
Xu
a and
Ming-Hua
Xu
*b
aDepartment of Chemistry, Innovative Drug Research Center, Shanghai University, Shanghai 200444, China
bState Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zuchongzhi Road, Shanghai 201203, China. E-mail: wenwen@simm.ac.cn; xumh@simm.ac.cn; Fax: +86 21-50807388
First published on 12th July 2016
Abstract
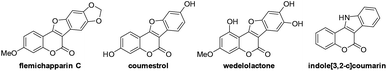
A palladium-catalyzed highly efficient and atom-economical intramolecular cross dehydrogenative coupling (CDC) reaction to access fused polyheterocycles containing a coumarin nucleus has been developed. A wide range of coumestans and indole[3,2-c]coumarins can be afforded in good to excellent yields (up to 99%). By taking advantage of this methodology, some biologically active molecules such as coumestrol and flemichapparin C can be facilely constructed in great yields.
Coumarins are widely present as key structures in a large variety of natural products and biologically active molecules.1 Among them, coumarin-based fused polyheterocycles such as coumestans (i.e. benzofuro[3,2-c]coumarins) and indole[3,2-c]coumarins are important pharmacophoric scaffolds with diverse biological applications and represent a valuable class of compounds in drug discovery. For example, flemichapparin C,2 coumestrol,3 and wedelolactone4 are naturally occurring coumestans exhibiting interesting estrogenic, antioxidant, antihepatotoxic and antitumor activities while artificially synthesized indole[3,2-c]coumarin5 was reported to be a potential reagent for anti-tumor angiogenesis (Fig. 1). Thus, great attention has been drawn towards the development of methodologies for the synthesis of coumestans and related compounds in the past few decades. Although considerable efforts have been devoted to achieve this synthesis, conventional methods generally require multi-step transformations under harsh reaction conditions, and often suffer from the limited scope of the starting materials and poor functional-group tolerance.6 Therefore, the development of new efficient methods for rapid construction of coumestan and its related compounds is of great importance and is highly desirable.
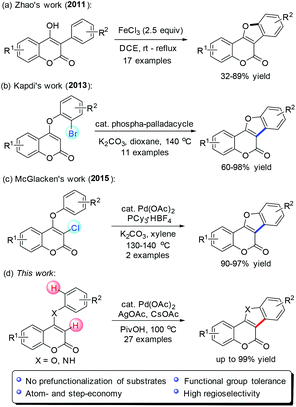
Transition-metal catalyzed C–H functionalization has received tremendous progress in recent years.7 However, highly practical processes to access coumestan-type heterocycles have rarely been realized with this protocol.8 In 2011, Zhao and co-workers reported a FeCl3-mediated direct intramolecular oxidative annulation of 4-hydroxy coumarins to attain coumestan analogues in moderate to good yields (Scheme 1a).9 However, substrates with several special functional groups on the C-3 substituted phenyl ring cannot be tolerated. Kapdi10 and McGlacken11 independently developed an intramolecular direct arylation approach using pre-halogen-functionalized 4-aryloxy coumarins as substrates (Scheme 1b and c). From the atom- and step-economical viewpoint, it is more desirable to conduct the ring construction via direct C–H functionalization without prefunctionalization.
Previously, we succeeded in the efficient assembly of pyrrolocoumarins and furocoumarins by palladium-catalyzed sequential coupling/cyclization reactions.12 In continuation of our interest in facile synthesis of coumarin-based polyheterocyclic compounds for drug discovery, we envisioned that the rapid construction of coumestans and indole[3,2-c]coumarins might be ideally achieved with an intramolecular oxidative cross dehydrogenative coupling (CDC)13 strategy through palladium-catalyzed selective activation of two neighboring C–H bonds of 4-aryloxy and 4-arylamino coumarins (Scheme 1d). Herein, we disclose our successful development of such a promising intramolecular CDC approach, providing straightforward access to a wide variety of coumestans and indole[3,2-c]coumarins from readily available starting materials.14
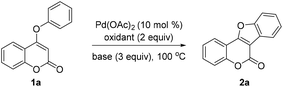
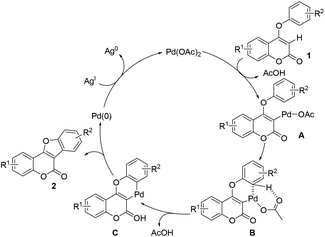
Inspired by Glorius's work on oxidative coupling of two C–H bonds within N-aryl-enamines,15 we initially performed the reaction of 4-aryloxy coumarin 1a in the presence of 10 mol% of Pd(OAc)2, 3 equiv. of K2CO3, and 3 equiv. of Cu(OAc)2, in DMF at 140 °C (Table 1, entry 1). Although the reaction did not proceed well under these conditions, we were pleased to detect the formation of trace amounts of the coumestan product 2a. Since acid may have a unique effect on the stabilization of the palladium intermediate in C–H activation reactions, we then turned to utilize PivOH as the solvent instead of DMF. Gratifyingly, the reaction proceeded to give the desired coumestan 2a in 32% yield (entry 2). Encouraged by these results, we commenced to examine the other parameters of the reaction. The choice of the oxidant is found to be crucial. Among the screened oxidants, AgOAc exhibited the best reactivity (entry 4), while K2S2O8 only led to trace amounts of the product (entry 3). Subsequently, the use of different bases was investigated. To our delight, CsOAc was found to be the most effective one, providing the product in a significantly improved 86% yield after 20 h (entry 6). Further reducing the amount of CsOAc to 2 equiv. did not affect the reaction yield (entry 7). Notably, the reaction could also proceed smoothly in the absence of a base, albeit with decreased yield (entry 8). Moreover, changing the solvent from PivOH to AcOH would lead to diminished yield (entry 8 vs. 9). Finally, the best results were obtained with a catalytic amount of Pd(OAc)2 (10 mol%), 2 equiv. of AgOAc as the oxidant, and 2 equiv. of CsOAc as the base in PivOH.
| Entry | Oxidant | Base | Solvent | Yieldb/% |
|---|---|---|---|---|
| a Reactions were conducted with 3a (0.20 mmol), Pd(OAc)2 (10 mol%), oxidant (2 equiv.) and base (3 equiv.) in 1.0 mL of solvent at 100 °C for 40 h. b Isolated yield. c The reaction time was 20 h. d 2 equiv. of base was utilized. | ||||
| 1 | Cu(OAc)2 | K2CO3 | DMF | Trace |
| 2 | Cu(OAc)2 | K2CO3 | PivOH | 32 |
| 3 | K2S2O8 | K2CO3 | PivOH | Trace |
| 4 | AgOAc | K2CO3 | PivOH | 69 |
| 5 | AgOAc | KOAc | PivOH | 83 |
| 6c | AgOAc | CsOAc | PivOH | 86 |
| 7c,d | AgOAc | CsOAc | PivOH | 86 |
| 8 | AgOAc | — | PivOH | 67 |
| 9 | AgOAc | — | AcOH | 56 |
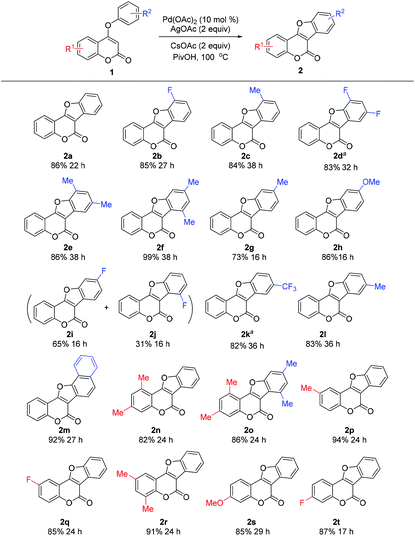
With the optimized conditions in hand, we then investigated the substrate scope of the reaction (Table 2). A wide range of 4-aryloxy coumarins bearing diverse electronic properties with substituents on either the phenyl ring of the coumarin nucleus or the C-4 substituted aryloxy smoothly participated in the intramolecular CDC reaction under palladium catalysis, providing a diverse set of coumestans generally in good to excellent yields (73–99%). The sterically encumbered coumarin substrates bearing ortho-substituents all worked well in the reaction, giving the products in high yields (2b–e, 2m). Notably, good to excellent regioselectivities were observed when meta-substituted substrates were employed. In the cases of meta-Me and meta-OMe substituted substrates, only sole isomer was attained in good yield (73% for 2g; 86% for 2h). Although a meta-F substituted substrate provided two regioisomers, the products were found to be separable by column chromatography and the total isolated yield (96%) remains high (65% for 2i; 31% for 2j). Gratifyingly, the reaction is tolerant of various electron-donating or electron-withdrawing substituents on the coumarin ring (2n–2t), demonstrating the excellent flexibility of this simple approach. To our knowledge, these products are difficult to prepare with known procedures and thus are rarely reported, especially those with electron-withdrawing groups.
| a 15 mol% Pd(OAc)2. |
|---|
|
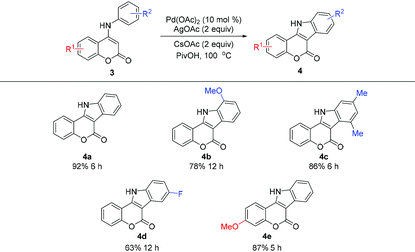
Having established the highly efficient intramolecular cross dehydrogenative coupling of 4-aryloxy coumarins, a preliminary investigation using 4-arylamino coumarins for a similar intramolecular oxidative C–H/C–H coupling was carried out (Table 3). Accordingly, 4-anilino coumarin 3a was chosen as the substrate. Under the aforementioned standard conditions, the reaction proceeded well and afforded the expected indole[3,2-c]coumarin product 4a in satisfactory yield (Table 3, 92% for 4a). Similarly, substrates containing different substituents are also tolerated, and indole[3,2-c]coumarins 4b–e were obtained in comparable yields. It is worth noting that this method is probably among the most practical synthetic pathways6h with no need of utilizing the toxic reagent NaN3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5,6a,b or prefunctionalization of substrates.6g,16 In particular, the ability of direct use of N-protection free substrates constitutes a remarkable advantage of this approach.
5,6a,b or prefunctionalization of substrates.6g,16 In particular, the ability of direct use of N-protection free substrates constitutes a remarkable advantage of this approach.
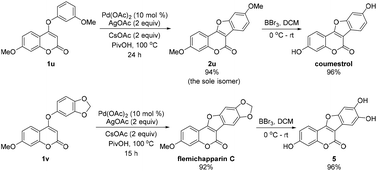
To highlight the synthetic utility, we applied this method in the synthesis of some biologically active coumestans (Scheme 2). When the meta-substituted coumarin 1u was subjected to the intramolecular CDC reaction, we were delighted to isolate the desired product 2u as the sole isomer in excellent yield (94% yield). After deprotection of the methoxy group, the natural product coumestrol was readily accessed in two steps with 90% total yield.17 In the other case, flemichapparin C was easily obtained in 92% yield through this CDC reaction with 1v as the substrate. Notably, a half-gram scale reaction (2 mmol of 1v) was successfully accomplished with comparable efficiency and yield (91%). To the best of our knowledge, this is among the shortest routes and best yields for the synthesis of coumestrol6c–e,8 and flemichapparin C.6f,g,11 Meanwhile, a biologically interesting molecule 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 can be facilely synthesized from flemichapparin C in excellent yield (96%) under BBr3/DCM conditions.
18 can be facilely synthesized from flemichapparin C in excellent yield (96%) under BBr3/DCM conditions.
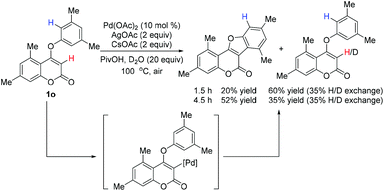
To understand the activation sequence of two C–H bonds of the coumarin substrate in cross dehydrogenative coupling, a preliminary H/D exchange experimental study was carried out.19 As shown in Scheme 3, treatment of 1o with Pd(OAc)2 in the presence of 20 equiv. of D2O resulted in a significant formation of the C-3 deuterium incorporated product (see the ESI† for 1H NMR analysis), which indicates that the initial palladation preferably occurs at the C-3 position (Scheme 4). Following the generation of the active Pd(II) species A, C–H activation may take place presumably through a concerted metalation–deprotonation mechanism to form intermediate C. Although it is difficult to elucidate the true pathway, the uniformly high reaction yields observed even with strong electron-withdrawing substituents on the aryloxy benzene ring might exclude the possibility of an SEAr mechanism. The subsequent reductive elimination furnishes the observed cyclized product 2 and finally the Pd(II) catalyst is regenerated by oxidation of Ag(I).
In summary, we have successfully developed an efficient Pd-catalyzed intramolecular oxidative cross dehydrogenative coupling reaction for easy access to a wide range of coumestans (benzofuro[3,2-c]coumarins) and indole[3,2-c]coumarins. High atom-economy efficiency, good functional group tolerance, and broad substrate scope make this protocol very practical. By means of this methodology, coumarin-fused natural products coumestrol and flemichapparin C as well as the biologically interesting compound 5 can be easily accessed in excellent yields through operationally simple procedures. These syntheses are probably among the most concise routes for coumestrol and flemichapparin C reported to date. Further mechanistic studies as well as application of this transformation in medicinal chemistry are under investigation.
Acknowledgements
Financial support from the National Natural Science Foundation of China (21325209, 81521005) and the Shanghai Municipal Committee of Science and Technology (14xd1404400) is greatly acknowledged.Notes and references
- (a) R. O'Kennedy and R. Zhorenes, Coumarins: Biology, Applications, Mode of Action, Wiley, Chichester, 1997; for reviews, see: Search PubMed; (b) J. R. S. Hoult and M. Paya, Gen. Pharmacol., 1996, 27, 713 CrossRef CAS PubMed; (c) D. L. Yu, M. Suzuki, L. Xie, S. L. Morris-Natschke and K. H. Lee, Med. Res. Rev., 2003, 23, 322 CrossRef CAS PubMed; (d) K. C. Fylaktakidou, D. J. Hadjipavlou-Litina, K. E. Litines and D. N. Nicolaides, Curr. Pharm. Des., 2004, 10, 3813 CrossRef CAS PubMed; (e) F. Borges, F. Roleira, N. Mihazes, L. Santana and E. Uriarte, Curr. Med. Chem., 2005, 12, 887 CrossRef CAS PubMed; (f) X.-S. Zhang, Z.-W. Li and Z.-J. Shi, Org. Chem. Front., 2014, 1, 44 RSC.
- (a) N. Adityachaudhury and P. K. Gupta, Phytochemistry, 1973, 12, 425 CrossRef; (b) N. Adityachaudhury and P. K. Gupta, Chem. Ind., 1970, 1113 Search PubMed.
- (a) E. M. Bickoff, A. N. Booth, R. L. Lyman, A. L. Livingston, C. R. Thompson and F. Deeds, Science, 1957, 126, 969 CAS; (b) E. M. Bickoff, A. N. Booth, R. L. Lyman, A. L. Livingston, C. R. Thompson and G. O. Kohler, J. Agric. Food Chem., 1958, 6, 536 CrossRef CAS; (c) N. Tsutsumi, Biol. Pharm. Bull., 1995, 18, 1012 CrossRef CAS PubMed.
- (a) T. R. Govindachari, K. Nagurajan and B. R. Pai, J. Chem. Soc., 1956, 629 RSC; (b) H. Wagner, B. Geyer, Y. Kiso, H. Hikino and G. S. Rao, Planta Med., 1986, 52, 370 CrossRef; (c) H. Wagner and B. Fessler, Planta Med., 1986, 52, 374 CrossRef; (d) P. A. Melo and C. L. Ownby, Toxicon, 1999, 37, 199 CrossRef CAS PubMed.
- W. Wang, Faming Zhuanli Shenqing, CN, 102321090, 2012 Search PubMed.
- Selected examples for the synthesis of coumestans, see: (a) W. Stadlbauer, A.-S. Karem and T. Kappe, Monatsh. Chem., 1987, 118, 81–89 CrossRef CAS; (b) Q. Ji, C. Yang, M. Wang and Y. Xie, Faming Zhuanli Shenqing, CN, 1966507, 2007 Search PubMed; (c) R. Laschober and T. Kappe, Synthesis, 1990, 387 CrossRef CAS; (d) N. Al-Maharik and N. P. Botting, Tetrahedron, 2004, 60, 1637 CrossRef CAS; (e) T. Yao, D. Yue and R. C. Larock, J. Org. Chem., 2005, 70, 9985 CrossRef CAS PubMed; (f) H. Leutbecher, J. Conrad, I. Klaiber and U. Beifuss, Synlett, 2005, 3126 CAS; (g) B. I. Kamara, E. V. Brandt and D. Ferreira, Tetrahedron, 1999, 55, 861 CrossRef CAS; (h) J. Wu, J. Lan, S. Guo and J. You, Org. Lett., 2014, 16, 5862 CrossRef CAS PubMed.
- For recent reviews on transition-metal-catalyzed C–H functionalization, see: (a) K. Gao and N. Yoshikai, Acc. Chem. Res., 2014, 47, 1208 CrossRef CAS PubMed; (b) L. Ackermann, J. Org. Chem., 2014, 79, 8948 CrossRef CAS PubMed; (c) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Rev., 2011, 111, 1293 CrossRef CAS PubMed; (d) C. Liu, H. Zhang, W. Shi and A. Lei, Chem. Rev., 2011, 111, 1780 CrossRef CAS PubMed; (e) S.-Y. Zhang, F.-M. Zhang and Y.-Q. Tu, Chem. Soc. Rev., 2011, 40, 1937 RSC; (f) D.-G. Yu, B.-J. Li and Z.-J. Shi, Tetrahedron, 2012, 68, 5130 CrossRef CAS; (g) B.-J. Lia and Z.-J. Shi, Chem. Soc. Rev., 2012, 41, 5588 RSC.
- U. A. Kshirsagar, R. Parnes, H. Goldshtein, R. Ofir, R. Zarivach and D. Pappo, Chem. – Eur. J., 2013, 19, 13575 CrossRef CAS PubMed.
- L. Tang, Y. Pang, Q. Yan, L. Shi, J. Huang, Y. Du and K. Zhao, J. Org. Chem., 2011, 76, 2744 CrossRef CAS PubMed.
- A. R. Kapdi, A. Karbelkar, M. Naik, S. Pednekar, C. Fischer, C. Schulzkec and M. Tromp, RSC Adv., 2013, 3, 20905 RSC.
- M.-T. Nolan, L. M. Pardo, A. M. Prendergast and G. P. McGlacken, J. Org. Chem., 2015, 80, 10904 CrossRef CAS PubMed.
- (a) L. Chen and M.-H. Xu, Adv. Synth. Catal., 2009, 351, 2005 CrossRef CAS; (b) L. Chen, Y. Li and M.-H. Xu, Org. Biomol. Chem., 2010, 8, 3073 RSC.
- For recent reviews, see: (a) C.-J. Li, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS PubMed; (b) C.-J. Li and W.-J. Yoo, Top. Curr. Chem., 2010, 292, 281 CAS; (c) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS PubMed; (d) Z. Shi and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 9220 CrossRef CAS PubMed; (e) A. Girard, T. Knauber and C.-J. Li, Angew. Chem., Int. Ed., 2014, 53, 74 CrossRef PubMed; (f) Y. Wu, J. Wang, F. Mao and F. Y. Kwong, Chem. – Asian J., 2014, 9, 26 CrossRef CAS PubMed.
- During the preparation of our manuscript, a related work for cyclization of 4-phenoxy-2-coumarins and 2-pyrones via C–H activation was published on the web on May 19: K. Mackey, L. M. Pardo, A. M. Prendergast, M.-T. Nolan, L. M. Bateman and G. P. McGlacken, Org. Lett., 2016, 18, 2540 CrossRef CAS PubMed.
- (a) S. Würtz, S. Rakshit, J. J. Neumann, T. Dröge and F. Glorius, Angew. Chem., Int. Ed., 2008, 47, 7230 CrossRef PubMed; (b) J. J. Neumann, S. Rakshit, T. Dröge, S. Würtz and F. Glorius, Chem. – Eur. J., 2011, 17, 7298 CrossRef CAS PubMed.
- (a) N. Thasana, R. Worayuthakarn, P. Kradanrat, E. Hohn, L. Young and S. Ruchirawat, J. Org. Chem., 2007, 72, 9379 CrossRef CAS PubMed; (b) C.-P. Chang, S. V. Pradiuldi and F.-E. Hong, Inorg. Chem. Commun., 2009, 12, 596 CrossRef CAS; (c) C. A. James, A. L. Coelho, M. Gevaert, P. Forgione and V. Snieckus, J. Org. Chem., 2009, 74, 4094 CrossRef CAS PubMed; (d) K. Ghosh, R. Karmakar and D. Mal, Eur. J. Org. Chem., 2013, 4037 CrossRef CAS; (e) P. Nealmongkola, K. Tangdenpaisalb, S. Sitthimonchaic, S. Ruchirawata and N. Thasana, Tetrahedron, 2013, 69, 9277 CrossRef.
- See the ESI† for synthetic details.
- (a) A. L. Livingston, S. C. Witt, R. E. Lundin and E. M. Bickoff, J. Org. Chem., 1965, 30, 2353 CrossRef CAS; (b) S. M. Wong, S. Antus, A. Gottsegen, B. Fessler, G. S. Rao, J. Sonnenbichler and H. Wagner, Arzneim.-Forsch., 1988, 38, 661 CAS.
- (a) H. Esaki, F. Aoki, M. Umemura, M. Kato, T. Maegawa, Y. Monguchi and H. Sajiki, Chem. – Eur. J., 2007, 13, 4052 CrossRef CAS PubMed; (b) M. Min, Y. Kim and S. Hong, Chem. Commun., 2013, 49, 196 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and spectroscopic data of all new compounds (PDF). See DOI: 10.1039/c6qo00270f |
| This journal is © the Partner Organisations 2016 |