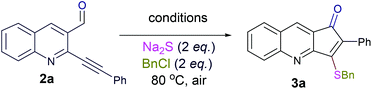Pd catalyzed facile synthesis of cyclopenta[b]quinolin-1-one via sequential Sonogashira coupling and annulation. An unusual mode of ring closure, using sulphur as a soft nucleophile†
Radhey M.
Singh
*,
Ritush
Kumar
,
Kishor Chandra
Bharadwaj
and
Tanu
Gupta
Department of Chemistry, Centre of Advanced Study, Institute of Science, Banaras Hindu University, Varanasi, India-221005. E-mail: rmohan@bhu.ac.in
First published on 5th July 2016
Abstract
Pd mediated one pot sequential Sonogashira coupling followed by annulation using o-alkynyl aldehyde is reported. Contrary to the established modes of ring closures, an unusual mode of the initial attack of sulphur across the triple bond occurs leading to a cascade of reactions. The protocol requires just single column chromatography, delivering cyclopenta[b]quinolin-1-one in high yields. Furthermore chemoselective transformations were carried out across annulated precursors.
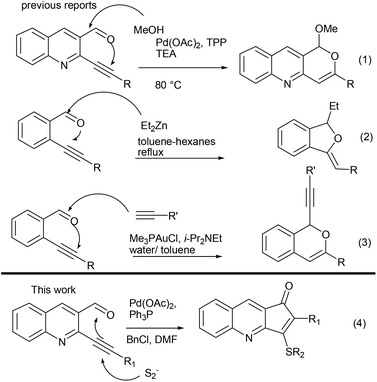
Aza aromatics are a privileged class of skeletons present in a number of natural products and biologically active molecules, with pyridine annulated fused rings being the most common architectural feature.1 In particular cyclopentenone fused pyridine and quinoline analogues have found frequent appearance in natural products along with a wide spectrum of biological activities.2 Prominent amongst them are anticancer,2a,d topoisomerase inhibitory activities,2b,h anti tuberculosis,2c DNA ligands2g and drug like properties.2e However, in spite of having such activities, less synthetic attention has been given towards the development of protocols for construction of these important skeletons.3 Recently Gao has carried out photoinduced radical cyclization for synthesis of these scaffolds.3a Yao has developed reductive cyclization towards this end.3e Our group has also reported Pd-catalyzed synthesis of 1-oxo, 3-methyl-2,3-dihydro-cyclopenta[b]quinolin via intramolecular Heck reactions of 2-chloroquinolin-3-yl-(1-homoallyl)alcohols.3g With a prominent void in the development of efficient protocols for these skeletons, we became interested in developing a robust method utilizing our research interest in the area of coupling and cyclization reactions for synthesis of various pyridine/quinoline annulated molecules.4,5
ortho-Alkynyl aldehyde (Fig. 1) has emerged as a valuable synthon in recent years, for the development of a wide range of protocols towards synthesis of various cyclic skeletons. With dual functional groups in close proximity they serve as an ideal platform for various nucleophile triggered cascade reactions.6 Previously we have used 2-(alkynyl)quinoline-3-carbaldehydes as precursors for synthesis of pyrano-quinoline where methoxide acted as a hard nucleophile triggering the domino sequence for cyclization (eqn (1), Fig. 1).7a Contributions from the lab of Zhao & Wang7b (eqn (2)), Li7c (eqn (3)) and others6 have also led to the domino reaction initiated by a nucleophilic attack over aldehydes. However a soft nucleophile such as sulphur has not been studied. We envisioned that the sulphur anion may undergo initial attack across the C–C π bond, a soft electrophile rather than aldehydes, which could be trapped by a tethered carbonyl moiety (eqn (4)).
It is worth mentioning here that this would be contrary to the well-established predominant mode of initial attack where various nucleophiles have been added on aldehydes6 and other polarized functional groups such as imines,8 nitriles9 and Michael acceptors10 followed by cyclization on triple bonds.
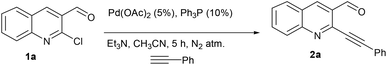
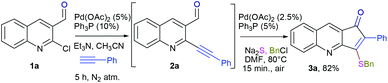
Development of protocols which leads to rapid synthesis of the desired product with a minimum number of steps involved has been a central theme in green chemistry. The advantages offered are obvious in terms of cost and economical concerns. Herein we report the palladium catalyzed synthesis of the cyclopenta[b]quinolin-1-one skeleton via sequential Sonogashira coupling and annulation. The protocol involves just single column chromatography (even including the synthesis of precursor)11 along with different substitution patterns. We initiated our studies with the synthesis of the required precursor via Sonogashira coupling (Scheme 1).12 Initially, we examined the palladium-catalyzed reaction of 2a with Na2S.7a
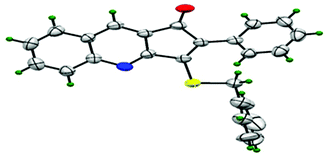
Thus, substrate 2a (0.50 mmol) was treated with 10 mol% of Pd(OAc)2, 20 mol% of PPh3 and 2 eq. Na2S, in methanol at 80 °C under an open atmosphere followed by quenching with benzyl chloride (Table 1, entry 1). The isolated product was characterized as 3-benzylsulfanyl-2-phenyl-cyclopenta[b]quinolin-1-ones 3a from its spectral and analytical data and furthermore unambiguously confirmed by single crystal X-ray analysis (Fig. 2).13
| Entry | Catalyst (mol%) | Ligand (mol%) | Solvent | Time (min) | Yielda (%) |
|---|---|---|---|---|---|
| a All reactions were carried out using 0.5 mmol of 2a. Isolated yield. b Reaction at 60 °C. c Reaction at 100 °C. | |||||
| 1 | Pd(OAc)2 (10) | Ph3P (20) | MeOH | 90 | 45 |
| 2 | Pd(OAc)2 (5) | Ph3P (10) | MeOH | 90 | 56 |
| 3 | Pd(OAc)2 (5) | Ph3P (10) | DMF | 15 | 82 |
| 4 | Pd(OAc) 2 (2.5) | Ph 3 P (5) | DMF | 15 | 88 |
| 5 | Pd(OAc)2 (1.0) | Ph3P (2.5) | DMF | 30 | 72 |
| 6b | Pd(OAc)2 (2.5) | Ph3P (5) | DMF | 15 | 42 |
| 7b | Pd(OAc)2 (2.5) | Ph3P (5) | DMF | 45 | 72 |
| 8c | Pd(OAc)2 (2.5) | Ph3P (5) | DMF | 15 | 60 |
| 9 | PdCl2 (2.5) | Ph3P (5) | DMF | 30 | 70 |
| 10 | Pd(dba)2 (2.5) | Ph3P (5) | DMF | 55 | 65 |
| 11 | Pd(OAc)2 (2.5) | Cy3P (5) | DMF | 65 | 69 |
| 12 | Pd(OAc)2 (2.5) | BINAP (2.5) | DMF | 40 | 55 |
| 13 | Pd(OAc)2 (2.5) | BINAP (5) | DMF | 40 | 58 |
| 14 | Pd(OAc)2 (2.5) | TMEDA (5) | DMF | 90 | 50 |
| 15 | Pd(OAc)2 (2.5) | Ph3P (5) | CH3CN | 60 | 60 |
| 16 | Pd(OAc)2 (2.5) | Ph3P (5) | THF | 75 | 60 |
| 17 | Pd(OAc)2 (2.5) | Ph3P (5) | Dioxane | 70 | 62 |
| 18 | Pd(OAc)2 (2.5) | Ph3P (5) | DMSO | 50 | 72 |
| 19 | CuI (2.5) | Ph3P (5) | DMF | 45 | — |
| 20 | AgOAc (2.5) | Ph3P (5) | DMF | 30 | — |
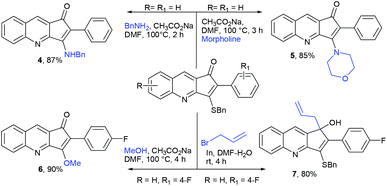
The formation of this product revealed that the preferred attack of sulphide takes over the alkyne rather than the carbonyl. This was rewarding as it leads to a new trend of cyclization and also delivered the highly substituted fused cyclopentenone annulated quinoline derivative. Inspired by the new result, we next considered finding optimal conditions. The results are summarized in Table 1. Lowering the mol% of Pd(OAc)2 and Ph3P to 5% and 10% respectively resulted in an increase of yield (56%, entry 2). Changing the solvent from methanol to DMF, the reaction was completed in 15 minutes along with enhanced yield to 82% (entry 3). On further decreasing the mol% ratio of the catalyst and ligand to 2.5% and 5% respectively, the yield of 3a further increased to 88% (entry 4). However further reduction in loading was not helpful (entry 5). Decreasing temperature leads to longer reaction times and decrease in yield (entries 6 & 7). Increasing reaction temperature lowered the yields (entry 8). Other available palladium catalysts such as PdCl2 and Pd(dba)2 were found less effective (entries 9 and 10). Ligands such as Cy3P, bidentate BINAP and nitrogen ligand TMEDA were also examined with Pd(OAc)2 (entries 11–14), but were found less effective than PPh3. The influence of other solvents such as CH3CN, THF, dioxane and DMSO (entries 15–18) was found less effective than DMF. Use of other metals such as CuI and AgOAc, didn't lead to product formation (entries 19 & 20). This could be attributed to the better electrophilic character of the palladium complex. Thus, the combination of 0.5 mmol of 2a with 2.5 mol% of Pd(OAc)2, 5 mol% of Ph3P, 2 eq. each of sodium sulphide and benzyl chloride in DMF at 80 °C, under an aerobic atmosphere was identified as the optimal reaction conditions (entry 4). As the Sonogashira and annulation steps were Pd catalyzed, we envisioned the possibility of a one pot sequence of both reactions. This would save time and solvent and avoid column chromatography purification of 2a. Thus, a mixture of 1a (0.5 mmol) and phenylacetylene (0.6 mmol) was treated with 5 mol% of Pd(OAc)2, 10 mol% of PPh3 and 2 eq. Et3N in acetonitrile at 80 °C for 5 h under a N2 atm. Subsequently volatiles were removed under reduced pressure. DMF along with 2.5 mol% of Pd(OAc)2, 5 mol% of Ph3P and 2 eq. of Na2S were added to the reaction mixture and it was heated at 80 °C under an open atm for 15 minutes. Quenching with 2 eq. of benzyl chloride, led to the formation of 3a in 82% overall yield (Scheme 2). With the one-pot optimal reaction conditions in hand, we next examined the generality of this reaction. Initially we extended the scope to various alkynes (Table 2). All reactions proceeded smoothly and were completed in 10–25 min. The corresponding cyclized products 3a–3e were obtained in 70–81% yields. No significant variation in the yields was found with phenylacetylene bearing electron donating or electron withdrawing groups. 3-Ethynyl-thiophene also afforded the cyclized product 3e in 70% yield. The generality of the reaction was further examined with various quinoline precursors. All the reactions proceeded smoothly and were completed in 10–25 min affording the corresponding cyclized products 3f–3k in 68–79% yields (Table 2). The reaction was also examined on the pyridine precursor which afforded the cyclized product 3l in 80% yield. Next, we extended the reaction scope by using different alkyl halides along with sodium sulphide. The reactions proceeded smoothly and afforded the product in good yields 3m–3r. Furthermore, quenching of the reaction with water instead of alkyl halide gave 3s in 72% yield. With the presence of multifunctional groups in cyclopentenone-fused quinoline 3, we further explored the possible transformation of these functionalities (Scheme 3). The 3-benzylsulfanyl group in 3 could be substituted by nucleophiles such as amines (primary and secondary) and alcohols to afford products 4, 5 and 6 in 87%, 85%, and 90% yields respectively. Similarly, the carbonyl group could also be converted to a tertiary alcohol using the Barbier reaction and afforded the allylated product 7 in 80% yield. It is worth mentioning here that in spite of different reactive sites on the periphery of the ring, all the transformations were chemoselective and delivered the products in high yields.
| a (i) Sonogashira coupling: 5 mol% Pd(OAc)2, 10 mol% Ph3P, 2 eq. Et3N, CH3CN, 80 °C, N2 atm; (ii) annulation: 2.5 mol% Pd(OAc)2, 5 mol% Ph3P, 2 eq. each of Na2S and R′′X, DMF, 80 °C, air. (iii) Overall yield over 2 steps and reaction time for each step are mentioned respectively. |
|---|
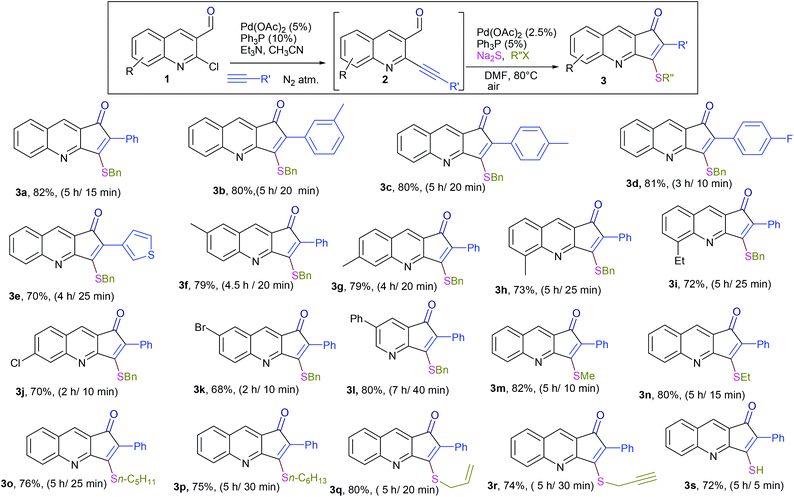
|
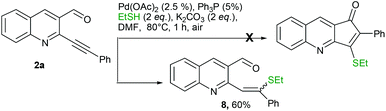
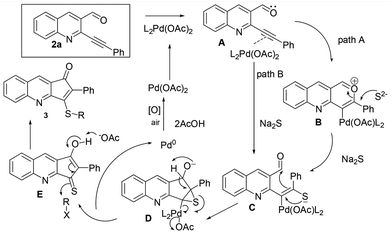
With a view of gaining further understanding about the path of the reaction, 2a was treated with ethane thiol and K2CO3 under optimized conditions. The reaction was completed in 1 h, however the isolated product didn't turn out to be a cyclized product. Instead it was characterized as 8 (Scheme 4). Lack of formation of the cyclized product suggested that the reaction initially proceeded via addition across triple bonds in Michael fashion which failed to further cyclize.14 A possible mechanism of the reaction is depicted in Fig. 3. Initial coordination of the triple bond with Pd(OAc)2 leads to activation. This could undergo nucleophilic attack either by aldehydic oxygen followed by the sulphide anion15 to give C (path A) or directly via the sulphide anion to give C (path B). Subsequent thiirane formation followed by nucleophilic attack on aldehydes gives D. Proton transfer followed by double bond formation and thiirane ring opening leads to expulsion of Pd0 which upon aerial oxidation gets converted to Pd(II). Finally proton abstraction and quenching with RX give the required product.
In conclusion, an efficient methodology has been developed for the synthesis of highly functionalized 3-alkylsulfanyl-2-aryl-cyclopenta[b]quinolin-1-ones via one pot two step sequential palladium-catalyzed Sonogashira coupling and annulation reactions as key steps. Furthermore, the synthetic potential of the annulated product was demonstrated by several chemoselective transformations.
Acknowledgements
RK and TG are thankful to UGC for the fellowship. KCB is thankful to DST for the fellowship. RMS is thankful to CSIR for funding (02(0073)/12EMR-II) and director CBMR Lucknow for the HRMS spectra. The authors are thankful to Deepak Joshi for solving crystallographic data. RMS is thankful to Prof. Y. D. Vankar, IIT Kanpur for fruitful suggestions in mechanism.Notes and references
- (a) E. Pan, S. Cao, P. J. Brodie, M. W. Callmander, R. Randrianaivo, S. Rakotonandrasana, E. Rakotobe, V. E. Rasamison, K. TenDyke, Y. Shen, E. M. Suh and D. G. I. Kingston, J. Nat. Prod., 2011, 74, 1169 CrossRef CAS PubMed; (b) M.-H. Yan, P. Cheng, Z.-Y. Jiang, Y.-B. Ma, X.-M. Zhang, F.-X. Zhang, L.-M. Yang, Y.-T. Zheng and J.-J. Chen, J. Nat. Prod., 2008, 71, 760 CrossRef CAS PubMed; (c) C. Dan, Y. Zhou, D. Ye, S. Peng, L. Ding, M. L. Gross and S. X. Qiu, Org. Lett., 2007, 9, 1813 CrossRef CAS PubMed; (d) T.-S. Wu and F.-W. Lin, J. Nat. Prod., 2001, 64, 1404 CrossRef CAS PubMed.
- (a) L. Goswami, S. Gogoi, J. Gogoi, R. K. Boruah, R. C. Boruah and P. Gogoi, ACS Comb. Sci., 2016, 18, 253 CrossRef CAS PubMed; (b) E. Kiselev, D. Sooryakumar, K. Agama, M. Cushman and Y. Pommier, J. Med. Chem., 2014, 57, 1289 CrossRef CAS PubMed; (c) G. C. Muscia, G. Y. Buldain and S. E. Asís, Eur. J. Med. Chem., 2014, 73, 243 CrossRef CAS PubMed; (d) S. Chakrabarty, M. S. Croft, M. G. Marko and G. Moyna, Bioorg. Med. Chem., 2013, 21, 1143 CrossRef CAS PubMed; (e) D. Camp, R. A. Davis, M. Campitelli, J. Ebdon and R. J. Quinn, J. Nat. Prod., 2012, 75, 72 CrossRef CAS PubMed; (f) R. Miri, O. Firuzi, P. Peymani, M. Zamani, A. R. Mehdipour, Z. Heydari, M. M. Farahani and A. Shafiee, Chem. Biol. Drug Des., 2012, 79, 68 CrossRef CAS PubMed; (g) N. Malecki, P. Carato, B. Rigo, J.-F. Goossens, R. Houssin, C. Bailly and J.-P. Hénichart, Bioorg. Med. Chem., 2004, 12, 641 CrossRef CAS PubMed; (h) L. W. Deady, A. J. Kaye, G. J. Finlay, B. C. Baguley and W. A. Denny, J. Med. Chem., 1997, 40, 2040 CrossRef CAS PubMed; (i) Y.-C. Wu and C.-Y. Duh, J. Nat. Prod., 1990, 5, 1327 CrossRef.
- (a) S. Cai, Z. Xiao, J. Ou, Y. Shi and S. Gao, Org. Chem. Front., 2016, 3, 354 RSC; (b) M. Zheng, P. Chen, W. Wu and H. Jiang, Chem. Commun., 2016, 52, 84 RSC; (c) J. K. Laha, K. P. Jethava and S. Patel, Org. Lett., 2015, 17, 5890 CrossRef CAS PubMed; (d) N. Marquise, V. Dorcet, F. Chevallier and F. Mongin, Org. Biomol. Chem., 2014, 12, 8138 RSC; (e) R. R. Rajawinslin, S. D. Gawande, V. Kavala, Y.-H. Huang, C.-W. Kuo, T.-S. Kuo, M.-L. Chen, C.-H. He and C.-F. Yao, RSC Adv., 2014, 4, 37806 RSC; (f) S. Dhara, A. A. S. Nandi, S. Baitalik and J. K. Ray, Tetrahedron Lett., 2013, 54, 63 CrossRef CAS; (g) R. M. Singh, A. Chandra, N. Sharma, B. Singh and R. Kumar, Tetrahedron, 2012, 68, 9206 CrossRef CAS; (h) G. Oppermann, M. Stranberg, H. W. Moore, E. Schaumann and G. Adiwidjaja, Synthesis, 2010, 2027 CAS.
- (a) J. B. Singh, K. C. Bharadwaj, T. Gupta and R. M. Singh, RSC Adv., 2016, 6, 26993 RSC; (b) M. Asthana, J. B. Singh and R. M. Singh, Tetrahedron Lett., 2016, 57, 615 CrossRef CAS; (c) M. Asthana, R. Kumar, T. Gupta and R. M. Singh, Tetrahedron Lett., 2015, 56, 907 CrossRef CAS; (d) M. Asthana, N. Sharma, R. Kumar, J. B. Singh and R. M. Singh, Tetrahedron Lett., 2014, 55, 4378 CrossRef CAS; (e) M. Asthana, N. Sharma and R. M. Singh, Tetrahedron, 2014, 70, 7996 CrossRef CAS; (f) D. Nandini, M. Asthana, T. Gupta, R. P. Singh and R. M. Singh, Tetrahedron, 2014, 70, 8592 CrossRef CAS; (g) R. M. Singh, K. C. Bharadwaj and D. K. Tiwari, Beilstein J. Org. Chem., 2014, 10, 2975 CrossRef PubMed; (h) D. Nandini, M. Asthana, K. Mishra, R. P. Singh and R. M. Singh, Tetrahedron Lett., 2014, 55, 6257 CrossRef CAS.
- (a) P. Zhao, Y. Liu and C. Xi, Org. Lett., 2015, 17, 4388 CrossRef CAS PubMed; (b) Y. Wang, C. Chen, S. Zhang, Z. Lou, X. Su, L. Wen and M. Li, Org. Lett., 2013, 15, 4794 CrossRef CAS PubMed; (c) Y. Wang, C. Chen, J. Peng and M. Li, Angew. Chem., Int. Ed., 2013, 52, 5323 CrossRef CAS PubMed.
- (a) M. D. Acqua, B. Castano, C. Cecchini, T. Pedrazzini, V. Pirovano, E. Rossi, A. Caselli and G. Abbiati, J. Org. Chem., 2014, 79, 3494 CrossRef PubMed; (b) D. Malhotra, L.-P. Liu, M. S. Mashuta and G. B. Hammond, Chem. – Eur. J., 2013, 19, 4043 CrossRef CAS PubMed; (c) E. Parker, N. Leconte, T. Godet and P. Belmont, Chem. Commun., 2011, 47, 343 RSC; (d) X. Bantreil, C. Vaxelaire, T. Godet, E. Parker, C. Sauer and P. Belmont, Org. Biomol. Chem., 2011, 9, 4831 RSC; (e) A. B. Beeler, S. Su, C. A. Singleton and J. A. Porco Jr., J. Am. Chem. Soc., 2007, 129, 1413 CrossRef CAS PubMed; (f) T. Godet, C. Vaxelaire, C. Michel, A. Milet and P. Belmont, Chem. – Eur. J., 2007, 13, 5632 CrossRef CAS PubMed; (g) N. T. Patil and Y. Yamamoto, J. Org. Chem., 2004, 69, 5139 CrossRef CAS PubMed.
- (a) A. Chandra, B. Singh, R. S. Khanna and R. M. Singh, J. Org. Chem., 2009, 74, 5664 CrossRef CAS PubMed; (b) Z. Chai, Z. F. Xie, X.-Y. Liu, G. Zhao and J.-D. Wang, J. Org. Chem., 2008, 73, 2947 CrossRef CAS PubMed; (c) X. Yao and C.-J. Li, Org. Lett., 2006, 8, 1953 CrossRef CAS PubMed.
- (a) Z. Guo, M. Cai, J. Jiang, L. Yang and W. Hu, Org. Lett., 2010, 12, 652 CrossRef CAS PubMed; (b) S. Obika, H. Kono, Y. Yasui, R. Yanada and Y. Takemoto, J. Org. Chem., 2007, 72, 4462 CrossRef CAS PubMed; (c) R. Yanada, S. Obika, H. Kono and Y. Takemoto, Angew. Chem., Int. Ed., 2006, 45, 3822 CrossRef CAS PubMed; (d) N. S. Asao, S. Y. S. T. Nogami and Y. Yamamoto, Angew. Chem., Int. Ed., 2005, 44, 5526 CrossRef CAS PubMed.
- (a) R. M. Singh, R. Kumar, N. Sharma and M. Asthana, Tetrahedron, 2013, 69, 9443 CrossRef CAS; (b) L.-M. Wei, C.-F. Lin and M.-J. Wu, Tetrahedron Lett., 2000, 41, 1215 CrossRef CAS; (c) R. C. Larock, Q. Tian and A. A. Pletnev, J. Am. Chem. Soc., 1999, 121, 3238 CrossRef CAS.
- (a) G. Qiu, Q. Ding, K. Gao, Y. Peng and J. Wu, ACS Comb. Sci., 2011, 13, 13 CrossRef CAS PubMed; (b) L. Liu, L. Wei, Y. Lu and J. Zhang, Chem. – Eur. J., 2010, 16, 11813 CrossRef CAS PubMed.
- (a) A. Srivastava and R. M. Singh, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2005, 44, 1868 Search PubMed.
- (a) D. Wang and S. Gao, Org. Chem. Front., 2014, 1, 556 RSC; (b) R. Chinchilla and C. Nájera, Chem. Soc. Rev., 2011, 40, 5084 RSC; (c) R. Chinchilla and C. Nájera, Chem. Rev., 2007, 107, 874 CrossRef CAS PubMed; (d) H. Doucet and J.-C. Hierso, Angew. Chem., Int. Ed., 2007, 46, 834 CrossRef CAS PubMed.
- CCDC no. 1046365.
- H. Tsukamoto, T. Ueno and Y. Kondo, Org. Lett., 2007, 9, 3033 CrossRef CAS PubMed.
- (a) I. Talbi, C. Alayrac, J.-F. Lohier, S. Touil and B. Witulski, Org. Lett., 2016, 18, 2656 CrossRef CAS PubMed; (b) S. Zheng, W. Huang, N. Gao, R. Cui, M. Zhang and X. Zhao, Chem. Commun., 2011, 47, 6969 RSC; (c) L.-L. Sun, C.-L. Deng, R.-Y. Tang and X.-G. Zhang, J. Org. Chem., 2011, 76, 7546 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterisation data and copies of 1H & 13C NMR spectra. CCDC 1046365. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00203j |
| This journal is © the Partner Organisations 2016 |