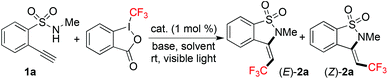Generation of benzosultams via trifluoromethylation of 2-ethynylbenzenesulfonamide under visible light†
Yuanchao
Xiang
a,
Yunyan
Kuang
*a and
Jie
Wu
*ab
aDepartment of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433, China. E-mail: jie_wu@fudan.edu.cn; yykuang@fudan.edu.cn
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China
First published on 18th May 2016
Abstract
Under visible light irradiation, 2-ethynylbenzenesulfonamides react with Togni's reagent in the presence of a photocatalyst leading to 3-(2,2,2-trifluoroethylidene)-2,3-dihydrobenzo[d]isothiazole 1,1-dioxides in good yields. This transformation proceeds efficiently at room temperature through a photo-initiated trifluoromethylation. The subsequent C–N bond formation produces the corresponding benzosultams.
Introduction
Currently, continuous efforts are being made towards the construction of nitrogen-containing heterocycles in the drug discovery process and for the studies of chemical genetics.1 Among the scaffolds of nitrogen-containing heterocycles, benzosultams have attracted our attention due to their importance in natural products and pharmaceuticals with diverse biological activities.2 Over the past decade, we have been interested in the generation of natural product-like compounds and their subsequent biological evaluations.3 During the studies, we identified that some hits with the core of benzosultam showed promising activities for inhibition of cervical carcinoma. It is known that the medicinal properties of drugs or leading compounds could often be significantly improved if the fluorine atom is incorporated into the molecules.4 Therefore, we initiated a program for the preparation of fluoro-substituted benzosultams, with an expectation to find more active compounds.The photo-induced organic transformations attracted our interest due to the efficiency of these methods and mild reaction conditions.5,6 Usually, a single electron transfer (SET) process is involved in photocatalysis, which promotes the completion of the reaction. The advantages and efficiency of photochemical reactions make them attractive in organic synthesis. Among the photo-induced transformations, trifluoromethylation under photoredox catalysis has made rapid progress.7–10 Usually, trifluoromethylation occurs in the presence of visible light and a photocatalyst at room temperature. In most cases, transition metals and organic molecules were employed as catalysts in trifluoromethylation. These photocatalysts were crucial to fulfill the photocatalytic cycles via SET processes. In general, the photocatalyst was stimulated to the excited state with visible light, which then provided an electron to the fluorinating reagent with the release of a CF3 radical. Prompted by the photoinduced trifluoromethylation,7–10 we envisioned that trifluoromethyl-substituted benzosultams would be accessed under suitable conditions. Recently, we reported an efficient synthesis of 4-((trifluoromethyl)thio)-2H-benzo[e][1,2]thiazine 1,1-dioxides through a BiCl3-promoted reaction of 2-(2-alkynyl)benzenesulfonamide with trifluoromethanesulfanylamide.11a We conceived that 2-(2-alkynyl)benzenesulfonamides could be utilized as the starting materials as well for the photoinduced trifluoromethylation. Thus, we started to explore the feasibility for the formation of trifluoromethyl-substituted benzosultams via trifluoromethylation of 2-(2-alkynyl)benzenesulfonamides under photocatalysis.
Results and discussion
Due to the easy availability, Togni's reagent7 was utilized as the source of trifluoromethylation. At the outset, the reaction of N-methyl-2-(phenylethynyl)benzenesulfonamide11 and Togni's reagent was studied by using Ir(ppy)3 as the photocatalyst under visible light. However, no reaction took place although different solvents and bases were screened. Since the addition of a trifluoromethyl radical to the triple bond would be the key step during the reaction process, we postulated that the steric hinderance of the alkyne in N-methyl-2-(phenylethynyl)benzenesulfonamide would retard the reaction. Thus, 2-ethynyl-N-methylbenzenesulfonamide 1a was used as a replacement for N-methyl-2-(phenylethynyl)benzenesulfonamide with Togni's reagent, and the reaction was re-explored. Initially, the reaction was catalyzed with Ir(ppy)3 (1 mol%) at 25 °C in MeCN under visible light irradiation in the presence of potassium carbonate as a base (Table 1, entry 1). To our delight, we observed the formation of desired products. The corresponding (Z)/(E)-3-(2,2,2-trifluoroethylidene)-2,3-dihydrobenzo[d]isothiazole 1,1-dioxides 2a were generated. These two compounds could be isolated, and the structures of (E)-2a and (Z)-2a were unambiguously identified via X-ray crystallography analysis (see the ESI†). Interestingly, only Ir(ppy)3 was effective as the photocatalyst, and the reaction failed to provide the expected products when Ru(bpy)3(PF6)2, eosin Y, or fluorescein was employed in the transformation (Table 1, entries 2–4). The presence of Ir(ppy)3 was essential, since no reaction occurred in a controlled experiment without the addition of a photocatalyst (data not shown in Table 1). Subsequently, different solvents were evaluated (Table 1, entries 5–12). When 1,4-dioxane was used as the solvent, the desired (E)-2a and (Z)-2a were produced in 20% and 12% yields, respectively (Table 1, entry 5). Only a trace amount of products was detected when the reaction was performed in DMF, MeOH, or Et2O. Compared with the results obtained in CH2Cl2, DCE, acetone, and EtOAc, acetonitrile was demonstrated as the best solvent of choice. Further survey of bases revealed that the reaction worked efficiently when NaHCO3 was used as the base, which provided the corresponding (E)-2a and (Z)-2a in 48% and 30% yields, respectively (Table 1, entries 13–21). The presence of other bases would result in inferior yields.| Entry | Cat. | Base | Solvent | Yieldb (%) | |
|---|---|---|---|---|---|
| (E)-2a | (Z)-2a | ||||
| a Reaction conditions: 2-ethynyl-N-methylbenzenesulfonamide 1a (0.2 mmol), Togni's reagent (1.2 equiv., 0.24 mmol), base (1.5 equiv., 0.3 mmol), photocatalyst (1 mol%), solvent (4.0 mL), N2, room temperature, under visible light irradiation (8W) for 14 h. b Determined by 19F NMR with the use of trifluoromethyl benzene as an internal standard. | |||||
| 1 | Ir(ppy)3 | K2CO3 | MeCN | 34 | 21 |
| 2 | Ru(bpy)3(PF6)2 | K2CO3 | MeCN | Trace | Trace |
| 3 | Eosin Y | K2CO3 | MeCN | Trace | Trace |
| 4 | Fluorescein | K2CO3 | MeCN | Trace | Trace |
| 5 | Ir(ppy)3 | K2CO3 | Dioxane | 20 | 12 |
| 6 | Ir(ppy)3 | K2CO3 | DMF | Trace | Trace |
| 7 | Ir(ppy)3 | K2CO3 | CH2Cl2 | 18 | 13 |
| 8 | Ir(ppy)3 | K2CO3 | MeOH | Trace | Trace |
| 9 | Ir(ppy)3 | K2CO3 | DCE | 20 | 12 |
| 10 | Ir(ppy)3 | K2CO3 | Acetone | 26 | 14 |
| 11 | Ir(ppy)3 | K2CO3 | Et2O | Trace | Trace |
| 12 | Ir(ppy)3 | K2CO3 | EtOAc | 28 | 9 |
| 13 | Ir(ppy)3 | KHCO3 | MeCN | 36 | 24 |
| 14 | Ir(ppy)3 | K3PO4 | MeCN | 37 | 22 |
| 15 | Ir(ppy)3 | Na2CO3 | MeCN | 27 | 15 |
| 16 | Ir(ppy)3 | NaHCO3 | MeCN | 48 | 30 |
| 17 | Ir(ppy)3 | DABCO | MeCN | 33 | 20 |
| 18 | Ir(ppy)3 | NaOAc | MeCN | 22 | 13 |
| 19 | Ir(ppy)3 | KOAc | MeCN | 20 | 11 |
| 20 | Ir(ppy)3 | Cs2CO3 | MeCN | 21 | 11 |
| 21 | Ir(ppy)3 | NaOH | MeCN | 12 | 7 |
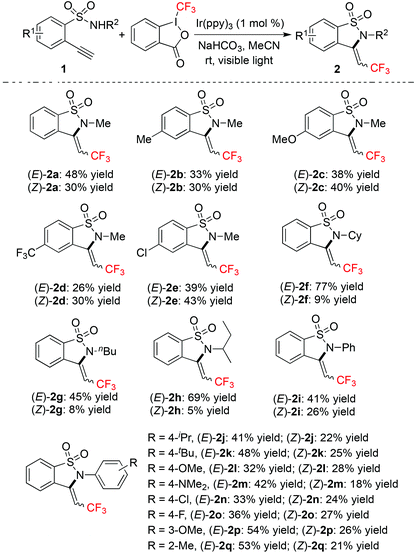
With the above optimized conditions in hand, we next explored the scope of the reaction of 2-ethynylbenzenesulfonamide 1 with Togni's reagent in the presence of a photocatalyst under visible light irradiation. The result is shown in Table 2. It was found that all reactions worked well, affording (E)-2 and (Z)-2 in totally good yields. All compounds of (E)-2 and (Z)-2 could be isolated easily via column chromatography. The generality of this approach was demonstrated, since different functional groups could be tolerated under the conditions. For example, the amino group could be compatible during the reaction process (product 2m).
| a Isolated yield based on 2-ethynylbenzenesulfonamide 1. |
|---|
|
It seemed that no big difference was displayed for the reactions of 2-ethynylbenzenesulfonamides 1 with electron-donating groups or electron-withdrawing groups attached to the aromatic ring. It is noteworthy that the halo group (F and Cl) could be retained during the reaction process. The ratio of products was influenced when the methyl group attached to the nitrogen atom was replaced by other groups. For instance, when 2-ethynyl-N-cyclohexylbenzenesulfonamide was used as the substrate, the corresponding products (E)-2f and (Z)-2f were obtained in 77% and 9% yields, respectively. Similar results were observed when the group attached to the nitrogen atom was changed to tert-butyl and sec-butyl groups.
Based on the photoinduced trifluoromethylation as reported previously,7 a plausible mechanism was proposed (Scheme 1). We postulated that the reaction proceeded initially through electron transfer from the photocatalyst, which was stimulated to the excited state by visible light. The fluorination reagent would accept an electron, thus resulting in the release of a CF3 radical. The addition of the CF3 radical to 2-ethynylbenzenesulfonamide 1 would generate the alkenyl radical C, which then underwent electron transfer to produce the alkenyl cation intermediate D. Then the subsequent intramolecular nucleophilic addition would provide 3-(2,2,2-trifluoroethylidene)-2,3-dihydrobenzo[d]isothiazole 1,1-dioxide 2.
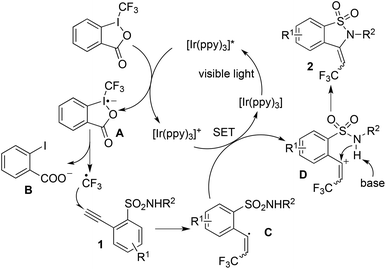 | ||
| Scheme 1 A proposed mechanism for the photo-induced trifluoromethylation of 2-ethynylbenzenesulfonamide 1 with Togni's reagent. | ||
Conclusions
In conclusion, we have described a photo-induced reaction of 2-ethynylbenzenesulfonamide with Togni's reagent in the presence of a photocatalyst. Under visible light irradiation, 3-(2,2,2-trifluoroethylidene)-2,3-dihydrobenzo[d]isothiazole 1,1-dioxides are generated in good yields. The (E)- and (Z)-isomers can be isolated easily. This transformation works efficiently at room temperature through photo-initiated electron transfer from the photocatalyst. The trifluoromethyl radical generated in situ is the key intermediate, which undergoes trifluoromethylation and the subsequent C–N bond formation leading to the corresponding benzosultams.Acknowledgements
Financial support from the National Natural Science Foundation of China (no. 21372046 and 21532001) is gratefully acknowledged.Notes and references
- Selected reviews on the synthesis of N-heterocycles: (a) A. Armstrong and J. C. Collins, Angew. Chem., Int. Ed., 2010, 49, 2282 CrossRef CAS PubMed; (b) A. R. Katritzky and S. Rachwal, Chem. Rev., 2010, 110, 1564 CrossRef CAS PubMed; (c) M. A. P. Martins, C. P. Frizzo, D. N. Moreira, L. Buriol and P. Machado, Chem. Rev., 2009, 109, 4140 CrossRef CAS PubMed; (d) M. Alvarez-Corral, M. Munoz-Dorado and I. Rodrıguez-Garcıa, Chem. Rev., 2008, 108, 3174 CrossRef CAS PubMed; (e) A. Minatti and K. Muniz, Chem. Soc. Rev., 2007, 36, 1142 RSC; (f) G. Zeni and R. C. Larock, Chem. Rev., 2006, 106, 4644 CrossRef CAS PubMed; (g) G. Qiu and J. Wu, Chem. Rec., 2016, 16, 19 CrossRef CAS PubMed; (h) G. Qiu, Y. Kuang and J. Wu, Adv. Synth. Catal., 2014, 356, 3483 CrossRef CAS.
- For selected examples, see: (a) L. Zhuang, J. S. Wai, M. W. Embrey, T. E. Fisher, M. S. Egbertson, L. S. Payne, L. P. Guare, J. P. Vacca, D. J. Hazuda, P. J. Felock, A. L. Wolfe, K. A. Stillmock, M. V. Witmer, G. Moyer, W. A. Schleif, L. J. Gabryelski, Y. M. Leonard, J. J. Lynch, S. R. Michelson and S. D. Young, J. Med. Chem., 2003, 46, 453 CrossRef CAS PubMed; (b) C. Valente, R. C. Guedes, R. Moreira, J. Iley, J. Gut and P. J. Rosenthal, Bioorg. Med. Chem. Lett., 2006, 16, 4115 CrossRef CAS PubMed; (c) M. Inagaki, T. Tsuri, H. Jyoyama, T. Ono, K. Yamada, M. Kobayashi, Y. Hori, A. Arimura, K. Yasui, K. Ohno, S. Kakudo, K. Koizumi, R. Suzuki, S. Kawai, M. Kato and S. Matsumoto, J. Med. Chem., 2000, 43, 2040 CrossRef CAS PubMed; (d) F. Brzozowski, F. Saczewski and N. Neamati, Bioorg. Med. Chem. Lett., 2006, 16, 5298 CrossRef PubMed; (e) G. J. Wells, M. Tao, K. A. Josef and R. Bihovsky, J. Med. Chem., 2001, 44, 3488 CrossRef CAS PubMed; (f) R. J. Cherney, R. Mo, D. T. Meyer, K. D. Hardman, R.-Q. Liu, M. B. Covington, M. Qian, Z. R. Wasserman, D. D. Christ, J. M. Trzaskos, R. C. Newton and C. P. Decicco, J. Med. Chem., 2004, 47, 2981 CrossRef CAS PubMed; (g) M. Bartholow, Top 200 Drugs of 2011. Pharmacy Times. http://www.pharmacytimes.com/publications/issue/2012/July2012/Top-200-Drugs-of-2011, accessed on Jan 9, 2013; (h) For a list of top drugs by year, see: http://cbc.arizona.edu/njardarson/group/top-pharmaceuticals-poster, accessed on Jan 9, 2013; (i) J. Drews, Science, 2000, 287, 1960 CrossRef CAS PubMed.
- For selected reviews, see: (a) H. Wang, Y. Kuang and J. Wu, Asian J. Org. Chem., 2012, 1, 302 CrossRef CAS; (b) L. He, H. Nie, G. Qiu, Y. Gao and J. Wu, Org. Biomol. Chem., 2014, 12, 9045 RSC; (c) G. Qiu, Q. Ding and J. Wu, Chem. Soc. Rev., 2013, 42, 5257 RSC; (d) Y. Luo, X. Pan, X. Yu and J. Wu, Chem. Soc. Rev., 2014, 43, 834 RSC; (e) G. Qiu and J. Wu, Synlett, 2014, 2703 CAS.
- (a) R. Filler and Y. Kobayashi, Biomedicinal Aspects of Fluorine Chemistry, Elsevier, Amsterdam, 1982 Search PubMed; (b) Fluorine in Bioorganic Chemistry, ed. J. T. Welch and S. Eswarakrishman, Wiley, New York, 1991 Search PubMed.
- For reviews, see: (a) T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527 CrossRef CAS PubMed; (b) D. M. Schultz and T. P. Yoon, Science, 2014, 343, 1239176 CrossRef PubMed; (c) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102 RSC; (d) J. Xuan and W.-J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828 CrossRef CAS PubMed; (e) L. Shi and W. Xia, Chem. Soc. Rev., 2012, 41, 7687 RSC; (f) Y.-M. Xi, H. Yi and A.-W. Lei, Org. Biomol. Chem., 2013, 11, 2387 RSC; (g) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed; (h) D. M. Schultz and T. P. Yoon, Science, 2014, 343, 985 CrossRef CAS PubMed; (i) M. N. Hopkinson, B. Sahoo, J.-L. Li and F. Glorius, Chem. – Eur. J., 2014, 20, 3874 CrossRef CAS PubMed; (j) J. Xuan, Z.-G. Zhang and W.-J. Xiao, Angew. Chem., Int. Ed., 2015, 54, 15632 CrossRef CAS PubMed; (k) J.-R. Chen, X.-Q. Hu, L.-Q. Lu and W.-J. Xiao, Chem. Soc. Rev., 2016, 45, 2044 RSC; (l) S. Protti, M. Fagnoni and D. Ravelli, ChemCatChem, 2015, 7, 1516 CrossRef CAS; (m) M. D. Karkas, B. S. Matsuura and C. R. J. Stephenson, Science, 2015, 349, 1285 CrossRef CAS PubMed; (n) R. Brimioulle, D. Lenhart, M. M. Maturi and T. Bach, Angew. Chem., Int. Ed., 2015, 54, 3872 CrossRef CAS PubMed; (o) D. Ravelli, M. Fagnoni and A. Albini, Chem. Soc. Rev., 2013, 42, 97 RSC; (p) T. P. Yoon, ACS Catal., 2013, 3, 895 CrossRef CAS PubMed; (q) M. A. Ischay and T. P. Yoon, Eur. J. Org. Chem., 2012, 3359 CrossRef CAS; (r) N. Hoffmann, ChemSusChem, 2012, 5, 352 CrossRef CAS PubMed; (s) J. W. Tucker and C. R. J. Stephenson, J. Org. Chem., 2012, 77, 1617 CrossRef CAS PubMed.
- For selected examples, see: (a) C. Uyeda, Y. Tan, G. C. Fu and J. C. Peters, J. Am. Chem. Soc., 2013, 135, 9548 CrossRef CAS PubMed; (b) S. E. Creutz, K. J. Lotito, G. C. Fu and J. C. Peters, Science, 2012, 338, 647 CrossRef CAS PubMed; (c) D. T. Ziegler, J. Choi, J. M. Muñoz-Molina, A. C. Bissember, J. C. Peters and G. C. Fu, J. Am. Chem. Soc., 2013, 135, 13107 CrossRef CAS PubMed; (d) J. A. Terrett, J. D. Cuthbertson, V. W. Shurtleff and D. W. C. MacMillan, Nature, 2015, 524, 330 CrossRef CAS PubMed; (e) C. C. Nawrat, C. R. Jamison, Y. Slutskyy, D. W. C. MacMillan and L. E. Overman, J. Am. Chem. Soc., 2015, 137, 11270 CrossRef CAS PubMed; (f) J. Jin and D. W. C. MacMillan, Nature, 2015, 525, 87 CrossRef CAS PubMed; (g) X.-Q. Hu, J.-R. Chen, Q. Wei, F.-L. Liu, Q.-H. Deng, A. M. Beauchemin and W.-J. Xiao, Angew. Chem., Int. Ed., 2014, 53, 12163 CrossRef CAS; (h) Y. Masuda, N. Ishida and M. Murakami, J. Am. Chem. Soc., 2015, 137, 14063 CrossRef CAS PubMed; (i) M. El Khatib, R. A. M. Serafim and G. A. Molander, Angew. Chem., Int. Ed., 2016, 55, 254 CrossRef CAS PubMed; (j) J. C. Tellis, D. N. Primer and G. A. Molander, Science, 2014, 345, 433 CrossRef CAS PubMed; (k) H.-Q. Do, S. Bachman, A. C. Bissember, J. C. Peters and G. C. Fu, J. Am. Chem. Soc., 2014, 136, 2162 CrossRef CAS PubMed; (l) A. C. Bissember, R. J. Lundgren, S. E. Creutz, J. C. Peters and G. C. Fu, Angew. Chem., Int. Ed., 2013, 52, 5129 CrossRef CAS PubMed; (m) W. Liu, L. Li and C.-J. Li, Nat. Commun., 2015, 6, 6526 CrossRef CAS PubMed; (n) L. Li, W. Liu, H. Zeng, X. Mu, G. Cosa, Z. Mi and C.-J. Li, J. Am. Chem. Soc., 2015, 137, 8328 CrossRef CAS PubMed; (o) Y. Masuda, N. Ishida and M. Murakami, J. Am. Chem. Soc., 2015, 137, 14063 CrossRef CAS PubMed.
- For selected examples, see: (a) S. P. Pitre, C. D. McTiernan, H. Ismaili and J. C. Scaiano, ACS Catal., 2014, 4, 2530 CrossRef CAS; (b) J. Xie, X. Yuan, A. Abdukader, C. Zhu and J. Ma, Org. Lett., 2014, 16, 1768 CrossRef CAS PubMed; (c) P. Xu, J. Xie, Q. Xue, C. Pan, Y. Cheng and C. Zhu, Chem. – Eur. J., 2013, 19, 14039 CrossRef CAS PubMed; (d) F. Gao, C. Yang, G. Gao, L. Zheng and W. Xia, Org. Lett., 2015, 17, 3478 CrossRef CAS PubMed; (e) R. Tomita, Y. Yasu, T. Koike and M. Akita, Angew. Chem., Int. Ed., 2014, 53, 7144 CrossRef CAS PubMed; (f) Y. Yasu, T. Koike and M. Akita, Chem. Commun., 2013, 49, 2037 RSC; (g) P. Xu, A. Abdukader, K. Hu, Y. Cheng and C. Zhu, Chem. Commun., 2014, 50, 2308 RSC; (h) S. Mizuta, K. M. Engle, S. Verhoog, O. Galicia-López, M. O'Duill, M. Médebielle, K. Wheelhouse, G. Rassias, A. L. Thompson and V. Gouverneur, Org. Lett., 2013, 15, 1250 CrossRef CAS PubMed; (i) R. Beniazza, F. Molton, C. Duboc, A. Tron, N. D. McClenaghan, D. Lastécouèresa and J.-M. Vincent, Chem. Commun., 2015, 51, 9571 RSC.
- For selected examples, see: (a) S. Mizuta, S. Verhoog, K. M. Engle, T. Khotavivattana, M. O'Duill, K. Wheelhouse, G. Rassias, M. Medebielle and V. Gouverneur, J. Am. Chem. Soc., 2013, 135, 2505 CrossRef CAS PubMed; (b) Y. Yasu, T. Koike and M. Akita, Org. Lett., 2013, 15, 2136 CrossRef CAS PubMed; (c) A. Carboni, G. Dagousset, E. Magnier and G. Masson, Chem. Commun., 2014, 50, 14197 RSC; (d) Q. Deng, J. Chen, Q. Wei, Q. Zhao, L. Lu and W. Xiao, Chem. Commun., 2015, 51, 3537 RSC; (e) N. Noto, K. Miyazawa, T. Koike and M. Akita, Org. Lett., 2015, 17, 3710 CrossRef CAS PubMed; (f) Y. Yasu, Y. Arai, R. Tomita, T. Koike and M. Akita, Org. Lett., 2014, 16, 780 CrossRef CAS PubMed; (g) Q. Wei, J. Chen, X. Hu, X. Yang, B. Lu and W. Xiao, Org. Lett., 2015, 17, 4464 CrossRef CAS PubMed; (h) Q. Lin, X. Xu and F. Qing, J. Org. Chem., 2014, 79, 10434 CrossRef CAS PubMed; (i) A. Sahoo, J. Li and F. Glorius, Angew. Chem., Int. Ed., 2015, 54, 11577 CrossRef PubMed; (j) S. H. Oh, Y. R. Malpani, N. Ha, Y. Jung and S. B. Han, Org. Lett., 2014, 16, 1310 CrossRef CAS PubMed; (k) X. Tang and W. R. Dolbier Jr., Angew. Chem., Int. Ed., 2015, 54, 4246 CrossRef CAS PubMed; (l) D. B. Bagal, G. Kachkovskyi, M. Knorn, T. Rawner, B. M. Bhanage and O. Reiser, Angew. Chem., Int. Ed., 2015, 54, 6999 CrossRef CAS PubMed.
- For recent examples, see: (a) D. Ravelli, S. Protti and M. Fagnoni, Chem. Rev. DOI:10.1021/acs.chemrev.5b00662; (b) Q. Lefebvre, N. Hoffmann and M. Rueping, Chem. Commun., 2016, 52, 2493 RSC; (c) B. Wang, D. Xiong and X. Ye, Org. Lett., 2015, 17, 5698 CrossRef CAS PubMed; (d) L. Zhu, L. Wang, B. Li, B. Fu, C. Zhang and W. Li, Chem. Commun., 2016, 52, 6371 RSC; (e) P. Gao, X. Song, X. Liu and Y. Liang, Chem. – Eur. J., 2015, 21, 7648 CrossRef CAS PubMed; (f) T. Besset, T. Poisson and X. Pannecoucke, Eur. J. Org. Chem., 2015, 2765 CrossRef CAS; (g) F. Wang, N. Zhu, P. Chen, J. Ye and G. Liu, Angew. Chem., Int. Ed., 2015, 54, 9356 CrossRef CAS PubMed; (h) Y. He, Q. Wang, L. Li, X. Liu, P. Xu and Y. Liang, Org. Lett., 2015, 17, 5188 CrossRef CAS PubMed; (i) R. Tomita, T. Koike and M. Akita, Angew. Chem., Int. Ed., 2015, 54, 12923 CrossRef CAS PubMed; (j) N. Iqbal, J. Jung, S. Park and E. Cho, Angew. Chem., Int. Ed., 2014, 53, 539 CrossRef CAS PubMed; (k) H. Hua, Y. He, Y. Qiu, Y. Li, B. Song and Y. Liang, Chem. – Eur. J., 2015, 21, 1468 CrossRef CAS PubMed; (l) J. Jacquet, S. Blanchard, E. Derat, M. Murr and L. Fensterbank, Chem. Sci., 2016, 7, 2030 RSC.
- For selected recent examples, see: (a) C. Ni, M. Hu and J. Hu, Chem. Rev., 2015, 115, 765 CrossRef CAS PubMed; (b) X. Liu, C. Xu, M. Wang and Q. Liu, Chem. Rev., 2015, 115, 683 CrossRef CAS PubMed; (c) C. Alonso, E. M. De Marigorta, G. Rubiales and F. Palacios, Chem. Rev., 2015, 115, 1847 CrossRef CAS PubMed; (d) E. Merino and C. Nevado, Chem. Soc. Rev., 2014, 43, 6598 RSC; (e) P. Yu, J.-S. Lin, L. Li, S.-C. Zheng, Y.-P. Xiong, L.-J. Zhao, B. Tan and X.-Y. Liu, Angew. Chem., Int. Ed., 2014, 53, 11890 CrossRef CAS PubMed; (f) C. Yu, N. Iqbal, S. Park and E. J. Cho, Chem. Commun., 2014, 50, 12884 RSC; (g) X.-L. Jiang, Z.-H. Chen, X.-H. Xu and F.-L. Qing, Org. Chem. Front., 2014, 1, 774 RSC; (h) C. Matheis, K. Jouvin and L. J. Goossen, Org. Lett., 2014, 16, 5984 CrossRef CAS PubMed; (i) D. J. Wilger, N. J. Gesmundo and D. A. Nicewicz, Chem. Sci., 2013, 4, 3160 RSC; (j) G. Landelle, A. Panossian, S. Pazenok, J. Vors and F. R. Leroux, Beilstein J. Org. Chem., 2013, 9, 2476 CrossRef PubMed; (k) T. Xu and G. Liu, Org. Lett., 2012, 14, 5416 CrossRef CAS PubMed; (l) X. Yang, T. Wu, R. J. Phipps and F. D. Toste, Chem. Rev., 2015, 115, 826 CrossRef CAS PubMed; (m) X.-H. Xu, K. Matsuzaki and N. Shibata, Chem. Rev., 2015, 115, 731 CrossRef CAS PubMed; (n) L. Chu and F.-L. Qing, Acc. Chem. Res., 2014, 47, 1513 CrossRef CAS PubMed; (o) Y. A. Serguchev, M. V. Ponomarenko and N. V. Ignaťev, J. Fluorine Chem., 2016, 185, 1 CrossRef CAS; (p) T. H. Rehm, Chem. Eng. Technol., 2016, 39, 66 CrossRef; (q) Y. Zeng, C. Ni and J. Hu, Chem. – Eur. J., 2016, 22, 3210 CrossRef CAS PubMed; (r) X. Xu and F. Qing, Curr. Org. Chem., 2015, 19, 1566 CrossRef CAS; (s) C. Ni, M. Hu and J. Hu, Chem. Rev., 2015, 115, 765 CrossRef CAS PubMed; (t) Y. Lu, C. Liu and Q. Chen, Curr. Org. Chem., 2015, 19, 1638 CrossRef CAS; (u) G. K. S. Prakash and J. Hu, Acc. Chem. Res., 2007, 40, 921 CrossRef CAS PubMed; (v) C. Ni and J. Hu, Synthesis, 2014, 842 Search PubMed; (w) X. Shen and J. Hu, Eur. J. Org. Chem., 2014, 4437 CrossRef CAS.
- (a) Q. Xiao, J. Sheng, Z. Chen and J. Wu, Chem. Commun., 2013, 49, 8647 RSC; (b) Q. Xiao, Y. Luo, Y. Yan, Q. Ding and J. Wu, RSC Adv., 2013, 3, 5779 RSC; (c) H. Wang, Y. Luo, X. Hou and J. Wu, Dalton Trans., 2013, 42, 4410 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure and related data. CCDC 1469995–1469996. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00120c |
| This journal is © the Partner Organisations 2016 |