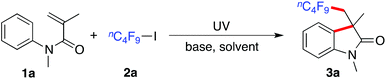A general route to fluorinated 3,3-disubstituted 2-oxindoles via a photoinduced radical cyclization of N-arylacrylamides under catalyst-free conditions†
Yuanyuan
An
a,
Yuewen
Li
a and
Jie
Wu
*ab
aDepartment of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433, China. E-mail: jie_wu@fudan.edu.cn
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Linglin Road, Shanghai 200032, China
First published on 3rd March 2016
Abstract
A catalyst-free radical cyclization of N-arylacrylamides with fluorinated alkyl iodides or the Togni reagent enabled by photoenergy is presented for the first time. Under ultraviolet irradiation, the generation of fluorinated 3,3-disubstituted 2-oxindoles proceeds smoothly without any metals or photoredox catalysts. The broad reaction scope is demonstrated with good functional group tolerance. During the process, fluorinated alkyl groups can be easily incorporated.
Introduction
So far, visible-light photocatalysis has attracted growing interest in organic transformations.1 Usually, reactive radicals or ionic species are involved during the reaction process, and photocatalysis is essential for the transformation. Recently, transition-metal-free reactions enabled by photoenergy were discovered.2 During the reaction process, no photocatalysis was needed and aryl/alkyl radicals could be generated easily from the corresponding aryl/alkyl halides. For example, aryl alkyne and alkyl iodide could be coupled in water under ultraviolet irradiation in the absence of a transition-metal.2a A photoinduced halogen exchange in aryl or vinyl halides could happen under metal-free conditions as well.2b It was demonstrated that these reactions proceeded through alkyl or aryl radicals from alkyl/aryl halides enabled by photoenergy. These results opened a new window for the coupling of aryl/alkyl halides under metal-free conditions, since these reactions were simply promoted by ultraviolet irradiation.Currently, the rapid generation of natural product-like small molecules is in high demand for studies of chemical genetics.3 In the meantime, much attention has been paid to the introduction of fluorinated substituents into privileged scaffolds, with the expectation to alter their physical, chemical, and biological properties.4 Consistent with our continuing interest in the library construction of natural product-like compounds and fluorinated molecules, we initiated a program for the synthesis of fluorinated isatin derivatives. Among the isatin derivatives, 3,3-disubstituted 2-oxindole attracted our attention due to its importance in pharmaceuticals.5,6 So far, the synthesis of trifluoromethyl-substituted 3,3-disubstituted 2-oxindoles have been reported by using the Togni reagent,7 TMSCF3,8 or CF3SO2Na9 as the fluoro source. The incorporation of other fluorinated groups into the skeleton was also developed,10a which proceeded through the reaction of N-arylacrylamides with fluoroalkylsulfonyl chlorides in the presence of photoredox catalysts. During the reaction process, fluorinated radicals were generated from RfSO2Cl by photoredox catalysis with the release of SO2. Very recently, fluoroalkyl iodides as the fluoro source were reported as well in the metal-catalyzed or AIBN-promoted process.10b,c Although the above transformations are efficient, metal catalysts are usually crucial in the reactions. Moreover, the process is not atom economical.
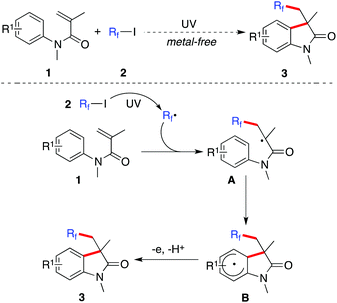
Prompted by the advancement of metal-free coupling reactions of alkyl/aryl halides enabled by photoenergy,2 we postulated that the fluorinated 3,3-disubstituted 2-oxindoles could be generated directly from the reaction of N-arylacrylamides 1 with fluoroalkyl iodides 2 under catalyst-free conditions. This hypothesis is shown in Scheme 1. We reasoned that the fluoroalkyl radical would be produced efficiently under ultraviolet irradiation. The subsequent addition of the fluoroalkyl radical to the double bond of N-arylacrylamide 1 would afford intermediate A. The further intramolecular attack of the aromatic ring would give rise to intermediate B. The subsequent aromatization would provide the corresponding fluorinated 3,3-disubstituted 2-oxindole 3. If this hypothesis is feasible, this proposed route would provide an efficient pathway to fluorinated heterocycles, since the transformation would proceed in the absence of metal catalysts or organocatalysts under mild conditions. Considering the importance of economic and environmental issues, this photochemistry is certainly a valuable alternative for the construction of fluorinated heterocycles, especially in the metal-free drug-discovery process.
 | ||
| Scheme 1 A proposed photoinduced radical cyclization of N-arylacrylamides 1 with fluoroalkyl iodides 2. | ||
Results and discussion
Our initial studies focused on the model reaction of N-methyl-N-phenylmethacrylamide 1a with n-nonafluorobutyl iodide 2a under ultraviolet irradiation at room temperature. The reaction occurred at 25 °C under ultraviolet irradiation (mercury lamp) at an intensity of 0.67 W cm−2 by using a standard photoreactor (see the ESI†). At the outset, the reaction was performed in the presence of sodium carbonate as the base in 1,2-dichloroethane (Table 1, entry 1). To our delight, the desired fluorinated 3,3-disubstituted 2-oxindole 3a was obtained in 24% yield. The yield was increased to 35% when 1,4-dioxane was used as a replacement (Table 1, entry 2). Further screening of solvents indicated that acetonitrile was the best choice, affording the expected product 3a in 73% yield (Table 1, entry 3). We also examined other bases (Table 1, entries 4–8), however, the results were inferior. Only a trace amount of product 3a was detected in a control experiment without the addition of base (Table 1, entry 9). The conversion was inert when the reaction occurred in the dark or under visible light irradiation (Table 1, entries 10 and 11). The advantages of this transformation are attractive: (1) mild conditions at room temperature; (2) without any metals or photoredox catalysts; (3) green process under ultraviolet irradiation.| Entry | Additive | Solvent | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: N-methyl-N-phenylmethacrylamide 1a (0.3 mmol), nC4F9I (0.3 mmol), base (0.3 mmol), solvent (4.0 mL), irradiation supplied by 600 W Hg light, rt, 12 h. b Isolated yield based on N-methyl-N-phenylmethacrylamide 1a. c The reaction occurred in the dark. d The reaction was performed under visible light irradiation. | |||
| 1 | Na2CO3 | DCE | 24 |
| 2 | Na2CO3 | 1,4-Dioxane | 35 |
| 3 | Na2CO3 | MeCN | 73 |
| 4 | NaOAc | MeCN | 47 |
| 5 | KOH | MeCN | 61 |
| 6 | NaHCO3 | MeCN | 60 |
| 7 | DBU | MeCN | 50 |
| 8 | DABCO | MeCN | 56 |
| 9 | — | MeCN | Trace |
| 10c | Na2CO3 | MeCN | n.d. |
| 11d | Na2CO3 | MeCN | n.d. |
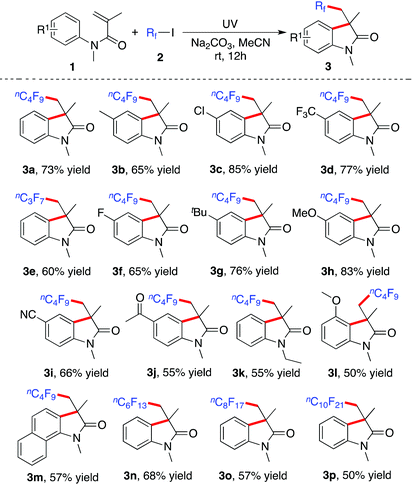
This promising result encouraged us to explore the scope of the reaction of N-arylacrylamides 1 with fluoroalkyl iodides 2. The result is shown in Table 2. Various N-arylacrylamides 1 reacted with fluoroalkyl iodides 2 efficiently, giving rise to the corresponding fluorinated 3,3-disubstituted 2-oxindoles 3 in moderate to good yields. A range of N-arylacrylamides 1 were examined. N-Arylacrylamides 1 with electron-withdrawing or electron-donating groups on the aromatic ring were all compatible under the standard conditions. Different functional groups were tolerated, including methyl, methoxy, chloro, fluoro, acetyl, and cyano groups. For instance, the cyano-substituted 2-oxindole 3i was obtained in 66% yield, while tert-butyl-substituted 2-oxindole 3g was produced in 76% yield. The chloro and fluoro atoms were remained in the corresponding products. Other fluoroalkyl iodides were employed as substrates as well in the transformation. For example, n-heptafluoropropyl iodide 2b was used in the reaction, leading to the corresponding product 3e in 60% yield.
| a Isolated yield based on N-arylmethacrylamide 1. |
|---|
|
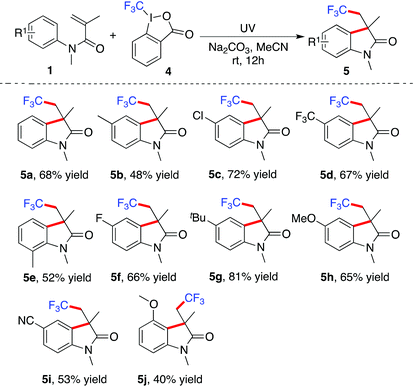
We further expanded the scope to the reaction of N-arylmethacrylamide 1 with Togni's reagent11 (Table 3). It has been of great synthetic interest for highly efficient and selective incorporation of the trifluoromethyl group into diverse skeletal structures.12 It was found that this photoinduced reaction of N-arylacrylamides 1 with Togni's reagent 4 also proceeded smoothly to afford the corresponding products as expected. The trifluoromethyl group could be easily installed in the scaffold of 2-oxindole. Again, the substrates featuring various functional groups including fluoro, chloro, trifluoromethyl, and cyano, worked well in this process.
| a Isolated yield based on N-arylmethacrylamide 1. |
|---|
|
Subsequently, this method was extended to N,N-diphenylmethacrylamide 6. As shown in Scheme 2, the reaction of N,N-diphenylmethacrylamide 6 with n-nonafluorobutyl iodide 2a or Togni's reagent worked well under standard conditions, giving rise to the corresponding product 7 or 8, respectively.
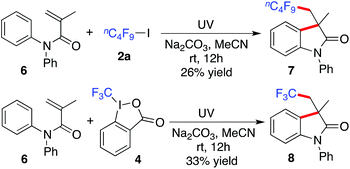 | ||
| Scheme 2 A photoinduced reaction of N,N-diphenylmethacrylamide 6 with n-nonafluorobutyl iodide 2a or Togni's reagent. | ||
Conclusions
In conclusion, we have described a catalyst-free radical cyclization of N-arylacrylamides with fluorinated alkyl iodides or the Togni reagent enabled by photoenergy. Under ultraviolet irradiation, the generation of fluorinated 3,3-disubstituted 2-oxindoles proceeds smoothly without any metals or photoredox catalysts. The broad reaction scope is demonstrated with good functional group tolerance. During the process, fluorinated alkyl groups can be easily incorporated. This approach provides an efficient pathway to fluorinated heterocycles, since the transformation would proceed in the absence of metal catalysts or organocatalysts under mild conditions. Considering the importance of economic and environmental issues, this photochemistry is certainly valuable. The advantages of this method including experimental ease, availability of the starting materials, and mild conditions will make this approach attractive for further applications.Acknowledgements
Financial support from the National Natural Science Foundation of China (no. 21372046 and 21532001) is gratefully acknowledged.Notes and references
- For reviews, see: (a) T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527 CrossRef CAS PubMed; (b) F. Teplý, Collect. Czech. Chem. Commun., 2011, 76, 859 CrossRef; (c) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102 RSC; (d) J. Xuan and W.-J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828 CrossRef CAS PubMed; (e) L. Shi and W.-J. Xia, Chem. Soc. Rev., 2012, 41, 7687 RSC; (f) Y.-M. Xi, H. Yi and A.-W. Lei, Org. Biomol. Chem., 2013, 11, 2387 RSC; (g) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed; (h) D. M. Schultz and T. P. Yoon, Science, 2014, 343, 985 CrossRef CAS PubMed; (i) M. N. Hopkinson, B. Sahoo, J.-L. Li and F. Glorius, Chem. – Eur. J., 2014, 20, 3874 CrossRef CAS PubMed; (j) J. Xuan, Z.-G. Zhang and W.-J. Xiao, Angew. Chem., Int. Ed., 2015, 54, 15632 CrossRef CAS PubMed; (k) J.-R. Chen, X.-Q. Hu, L.-Q. Lu and W.-J. Xiao, Chem. Soc. Rev., 2016 10.1039/C5CS00655D.
- (a) W. Liu, L. Li and C.-J. Li, Nat. Commun., 2015, 6, 6526 CrossRef CAS PubMed; (b) L. Li, W. Liu, H. Zeng, X. Mu, G. Cosa, Z. Mi and C.-J. Li, J. Am. Chem. Soc., 2015, 137, 8328 CrossRef CAS PubMed; (c) Y. Masuda, N. Ishida and M. Murakami, J. Am. Chem. Soc., 2015, 137, 14063 CrossRef CAS PubMed; (d) J. Ruch, A. Aubin, G. Erbland, A. Fortunato and J.-P. Goddard, Chem. Commun., 2016, 52, 2326 RSC.
- For selected examples, see: (a) S. L. Schreiber, Science, 2000, 287, 1964 CrossRef CAS PubMed; (b) P. Arya, D. T. H. Chou and M.-G. Baek, Angew. Chem., Int. Ed., 2001, 40, 339 CrossRef CAS; (c) M. D. Burk and S. L. Schreiber, Angew. Chem., Int. Ed., 2004, 43, 46 CrossRef PubMed; (d) D. S. Tan, Nat. Chem. Biol., 2005, 1, 74 CrossRef CAS PubMed; (e) D. P. Walsh and Y.-T. Chang, Chem. Rev., 2006, 106, 2476 CrossRef CAS PubMed; (f) C. Cordier, D. Morton, S. Murrison, A. Nelson and C. O'Leary-Steele, Nat. Prod. Rep., 2008, 25, 719 RSC; (g) S. L. Schreiber, Nature, 2009, 457, 153 CrossRef CAS PubMed; (h) W. R. J. D. Galloway, A. I. Llobet and D. R. Spring, Nat. Commun., 2010, 1, 80 Search PubMed; (i) S. Oh and S. B. Park, Chem. Commun., 2011, 47, 12754 RSC.
- (a) D. J. Hagan, Chem. Soc. Rev., 2008, 37, 308 RSC; (b) K. L. Kirk, Org. Process Res. Dev., 2008, 12, 305 CrossRef CAS; (c) P. Krisch, Modern Fluoroorganic Chemistry, Wiley-VHC, Weinheim, 2004 Search PubMed; (d) I. Ojima, Fluorine in Medicinal Chemistry and Chemical Biology, Wiley- Blackwell, Chichester, UK, 2009 Search PubMed; (e) K. Muller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef PubMed; (f) W. K. Hagmann, J. Med. Chem., 2008, 51, 4359 CrossRef CAS PubMed.
- (a) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748 CAS; (b) N. R. Ball-Jones, J. J. Badillo and A. K. Franz, Org. Biomol. Chem., 2012, 10, 5165 RSC; (c) J. J. Badillo, N. V. Hanhan and A. K. Franz, Curr. Opin. Drug Discovery Dev., 2010, 13, 758 CAS.
- Recent reviews for the synthesis of 3,3-disubstituted 2-oxindoles via the radical addition reaction involving N-arylacrylamides, see: (a) J.-R. Chen, X.-Y. Yu and W.-J. Xiao, Synthesis, 2015, 604 Search PubMed; (b) R.-J. Song, Y. Liu, Y.-X. Xie and J.-H. Li, Synthesis, 2015, 1195 CAS.
- (a) W. Fu, Y. Fu, C. Xu, S. Li and D. Zou, Eur. J. Org. Chem., 2014, 709 CrossRef CAS; (b) P. Xu, J. Xie, Q. Xue, C. Pan, Y. Cheng and Z. Cheng, Chem. – Eur. J., 2013, 19, 14039 CrossRef CAS PubMed; (c) H. Egami, R. Shimizu and M. Sodeoka, J. Fluorine Chem., 2013, 152, 51 CrossRef CAS.
- (a) L. Li, M. Deng, S. Zheng, Y. Xiong, B. Tan and X. Liu, Org. Lett., 2014, 16, 504 CrossRef CAS PubMed; (b) X. Mu, T. Wu, H. Wang, Y. Guo and G. Liu, J. Am. Chem. Soc., 2012, 134, 878 CrossRef CAS PubMed.
- (a) F. Yang, P. Klumphu, Y. Liang and B. H. Lipshutz, Chem. Commun., 2014, 50, 936 RSC; (b) J. Liu, S. Zhuang, Q. Gui, X. Chen, Z. Yang and T. Ze, Eur. J. Org. Chem., 2014, 3196 CrossRef CAS; (c) L. Zhang, Z. Li and Z. Liu, Org. Lett., 2014, 16, 3688 CrossRef CAS PubMed.
- (a) X.-J. Tang, C. S. Thomoson and W. R. Dolbier, Org. Lett., 2014, 16, 4594 CrossRef CAS PubMed; (b) H. Wang, L.-N. Guo and X.-H. Duan, J. Org. Chem., 2016, 81, 860 CrossRef CAS PubMed; (c) S. Tang, Z.-H. Li, M.-W. Wang, Z.-P. Li and R.-L. Sheng, Org. Biomol. Chem., 2015, 13, 5285 RSC.
- For selected reports, see: (a) Y. Li, M. Sun, H. Wang, Q. Tian and S. Yang, Angew. Chem., Int. Ed., 2013, 52, 3972 CrossRef CAS PubMed; (b) M. Zhou, R. Song, X. Ouyang, Y. Liu, W. Wei, G. Deng and J. Li, Chem. Sci., 2013, 4, 2690 RSC; (c) W. Wei, M. Zhou, J. Fan, W. Liu, R. Song, Y. Liu, M. Hu, P. Xie and J. Li, Angew. Chem., Int. Ed., 2013, 52, 3638 CrossRef CAS PubMed.
- For selected reviews for trifluoromethylation, see: (a) M. Schlosser, Angew. Chem., Int. Ed., 2006, 45, 5432 CrossRef CAS PubMed; (b) J.-A. Ma and D. Cahard, J. Fluorine Chem., 2007, 128, 975 CrossRef CAS; (c) J.-A. Ma and D. Cahard, Chem. Rev., 2008, 108, PR1 CrossRef CAS PubMed; (d) O. A. Tomashenko and V. Grushin, Chem. Rev., 2011, 111, 4475 CrossRef CAS PubMed; (e) A. Studer, Angew. Chem., Int. Ed., 2012, 51, 8950 CrossRef CAS PubMed; (f) X. Liu, C. Xu, M. Wang and Q. Liu, Chem. Rev., 2015, 115, 683 CrossRef CAS PubMed; (g) D. Browne, Angew. Chem., Int. Ed., 2014, 53, 1482 CrossRef CAS PubMed; (h) H. Liu, Z. Gu and X. Jiang, Adv. Synth. Catal., 2013, 355, 617 CrossRef CAS; (i) X. Liu and X. Wu, Synlett, 2013, 1882 CAS; (j) H. Egami and M. Sodeoka, Angew. Chem., Int. Ed., 2014, 53, 8294 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qo00055j |
| This journal is © the Partner Organisations 2016 |