Synthesis of fused isoquinolines via gold-catalyzed tandem alkyne amination/intramolecular O–H insertion†
Yuan
Pan
a,
Gui-Wei
Chen
b,
Cang-Hai
Shen
b,
Weimin
He
*a and
Long-Wu
Ye
*bc
aState Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China
bState Key Laboratory of Physical Chemistry of Solid Surfaces & The Key Laboratory for Chemical Biology of Fujian Province, Department of Chemistry, Xiamen University, Xiamen 361005, China. E-mail: longwuye@xmu.edu.cn
cState Key Laboratory of Organometallic Chemistry Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
First published on 12th February 2016
Abstract
A novel gold-catalyzed tandem alkyne amination/intramolecular O–H insertion has been developed. A variety of [1,4]oxazino[3,2-c]isoquinolines are readily accessed under mild reaction conditions by utilizing this strategy, thereby providing an efficient and practical route for the construction of synthetically useful fused isoquinolines.
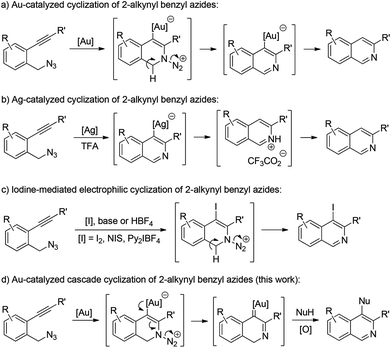
Isoquinolines are a commonly encountered structural motif in many natural products and bioactive compounds.1 Consequently, the development of new synthetic methodologies for their preparation remains an active research area.2 In recent years, transition metal- or iodine-promoted cyclization of readily available 2-alkynyl benzyl azides has emerged as one of the most efficient tools available for the construction of isoquinoline derivatives.3–6 For example, Yamamoto and co-workers reported a gold-catalyzed intramolecular cyclization of 2-alkynyl benzyl azides, allowing the facile synthesis of isoquinolines (Scheme 1a).3 Later, Liang and co-workers also described a general route for the construction of various isoquinolines via Ag-catalyzed cyclization of 2-alkynylbenzyl azides (Scheme 1b).4 Recently, elegant studies about the synthesis of isoquinolines from 2-alkynyl benzyl azides were reported by Yamamoto and co-workers by an iodine-mediated electrophilic cyclization reaction (Scheme 1c).5 Despite these significant achievements, the above cyclization has to be quenched by an electrophile, that is, the position 4 of the produced isoquinoline is limited to a hydrogen or halogen. Therefore, the exploration of novel and general methods for such a cyclization is highly desirable.
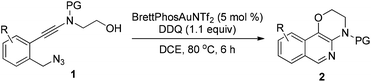
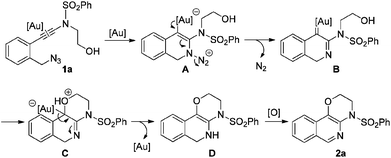
In our recent study on the ynamide chemistry,7–9 we disclosed that benzyl azides could serve as efficient nitrene-transfer reagents to react with ynamides for the generation of α-imino gold carbenes,10,11 leading to the highly site-selective synthesis of versatile 2-aminoindoles and 3-amino-β-carbolines.9a With these in mind, we envisioned that the above cyclization of 2-alkynylbenzyl azide might be trapped by a nucleophile through a gold-catalyzed amination-initiated tandem reaction involving an α-imino gold carbene as the intermediate (Scheme 1d). In this communication, we describe herein the realization of such a gold-catalyzed tandem alkyne amination/intramolecular O–H insertion, which provides ready access to valuable [1,4]oxazino[3,2-c]isoquinolines in generally good to excellent yields. Importantly, two fused six-membered rings have been built in one step.
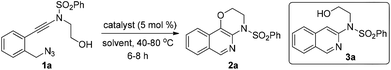
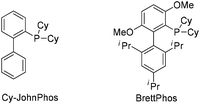
To test our hypothesis, (azido)ynamide 1a was prepared and treated with IPrAuNTf2 (5 mol%) in 1,2-dichloroethane (DCE) at 80 °C for 6 h. To our delight, 62% yield of the desired [1,4]oxazino[3,2-c]isoquinoline 2a was obtained, albeit with a minor isoquinoline 3a, which was formed similar to Yamamoto's protocol (Table 1, entry 1). Importantly, no Huisgen 1,3-dipolar cycloaddition product was observed in this case.3,4,5a,12 Subsequently, the influence of various gold catalysts bearing different ligands was examined (Table 1, entries 2–6), and BrettPhosAuNTf2 gave a slightly improved yield (Table 1, entry 5). PtCl2 was not effective in promoting this reaction (Table 1, entry 7) while no trace of the desired product 2a was detected by employing Zn(OTf)2 or Cu(OTf)2 as the catalyst (Table 1, entries 8 and 9). In addition, the use of other solvents, including toluene, PhCl and THF, led to a significantly decreased yield (Table 1, entries 10–12). Of note, the amount of byproduct 3a increased when the reaction was performed at a reduced temperature (Table 1, entry 13). Finally, different types of external oxidants, including O2, oxone, AgOAc and DDQ (2,3-dichloro-5,6-dicyano-1,4-benzoquinone), were investigated (Table 1, entries 14–17) and it was found that the use of DDQ gave the best result, which minimized the formation of the byproduct 3a (Table 1, entry 17).
| Entry | Catalyst | Conditions | Yieldb (%) | |
|---|---|---|---|---|
| 2a | 3a | |||
| a Reaction conditions: [1a] = 0.05 M. b Estimated by 1H NMR using diethyl phthalate as an internal reference. c Ar = 2,4-di-tert-butylphenyl. d >90% of 1a remained unreacted. e Using O2 (1 atm) as the oxidant. f Using oxone (2.0 equiv.) as the oxidant. g Using AgOAc (1.1 equiv.) as the oxidant. h Using DDQ (1.1 equiv.) as the oxidant. | ||||
| 1 | IPrAuNTf2 | DCE, 80 °C, 6 h | 62 | 25 |
| 2 | Ph3PAuNTf2 | DCE, 80 °C, 6 h | 49 | 19 |
| 3 | (4-CF3C6H4)3PAuNTf2 | DCE, 80 °C, 6 h | 56 | 38 |
| 4 | Cy-JohnPhosAuNTf2 | DCE, 80 °C, 6 h | 44 | 37 |
| 5 | BrettPhosAuNTf2 | DCE, 80 °C, 6 h | 68 | <10 |
| 6 | (ArO)3PAuNTf2c | DCE, 80 °C, 6 h | 51 | 36 |
| 7 | PtCl2 | Toluene, 80 °C, 6 h | 18 | 27 |
| 8d | Zn(OTf)2 (10 mol %) | DCE, 80 °C, 6 h | <5 | <5 |
| 9d | Cu(OTf)2 (10 mol %) | DCE, 80 °C, 6 h | <5 | <5 |
| 10 | BrettPhosAuNTf2 | Toluene, 80 °C, 6 h | 38 | 23 |
| 11 | BrettPhosAuNTf2 | PhCl, 80 °C, 6 h | 50 | 13 |
| 12 | BrettPhosAuNTf2 | THF, 80 °C, 6 h | 21 | 64 |
| 13 | BrettPhosAuNTf2 | DCE, 40 °C, 8 h | 40 | 25 |
| 14e | BrettPhosAuNTf2 | DCE, 80 °C, 6 h | 65 | 11 |
| 15f | BrettPhosAuNTf2 | DCE, 80 °C, 6 h | 51 | 13 |
| 16g | BrettPhosAuNTf2 | DCE, 80 °C, 6 h | 72 | <10 |
| 17h | BrettPhosAuNTf2 | DCE, 80 °C, 6 h | 91 | <5 |
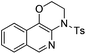
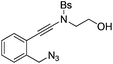
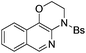
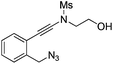
| Entry | Substrate | 1 | Product | 2 | Yield (%) |
|---|---|---|---|---|---|
| a Reactions run in vials; [1] = 0.05 M; isolated yields are reported. | |||||
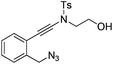
| 1 |
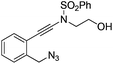
|
1a |
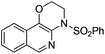
|
2a | 88 |
| 2 |
|
1b |
|
2b | 68 |
| 3 |
|
1c |
|
2c | 78 |
| 4 |
|
1d |
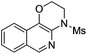
|
2d | 85 |
| 5 |
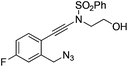
|
1e |
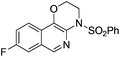
|
2e | 82 |
| 6 |
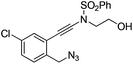
|
1f |
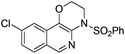
|
2f | 95 |
| 7 |
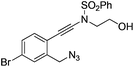
|
1g |
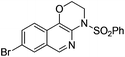
|
2g | 75 |
| 8 |
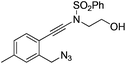
|
1h |
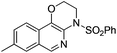
|
2h | 71 |
| 9 |
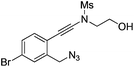
|
1i |
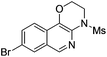
|
2i | 76 |
| 10 |
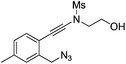
|
1j |
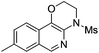
|
2j | 80 |
The reaction could also be extended to secondary alcohol-tethered ynamide 1k, derived from the commercially available (S)-(+)-1-amino-2-propanol, leading to the efficient formation of the corresponding optically active fused isoquinoline 2k in 93% yield (eqn (1)). In addition, (azido)ynamide 1l was also a suitable substrate for this tandem alkyne amination/O–H insertion reaction to furnish the corresponding isoquinoline fused with a seven membered ring, albeit with isoquinoline 3l as a significant byproduct (eqn (2)). Of note, our attempts to extend the reaction to tandem alkyne amination/intramolecular N–H insertion only gave a complicated mixture of products and no desired 2m was obtained (eqn (3)).
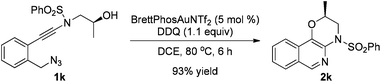 | (1) |
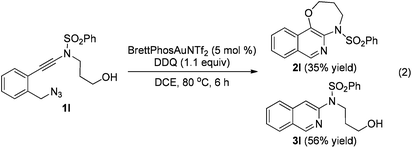 | (2) |
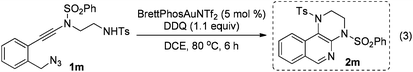 | (3) |
Finally, a plausible mechanism to rationalize this gold-catalyzed cascade reaction is presented in Scheme 2. Taking substrate 1a for example, an initial Au-catalyzed nucleophilic addition of the azide nitrogen onto the alkyne moiety in 1a, followed by extrusion of molecular nitrogen, generates the key α-imino gold carbene intermediate B. A subsequent intramolecular trapping of the α-imino gold carbenoid by the OH moiety affords the intermediate C, which undergoes further proton transfer/deauration/dehydrogenative oxidation to deliver the target fused isoquinoline 2a.
In summary, we have developed an efficient and practical method for the preparation of structurally diverse fused isoquinolines via an α-imino gold carbene intermediate. Importantly, fused six-membered rings have been built in one step, highlighting the power of this gold-catalyzed tandem sequence. Other notable features of this approach include readily available starting materials, high flexibility, the simple procedure and mild reaction conditions. Further investigations of this gold-catalyzed amination-initiated tandem reaction will be pursued in our laboratory.14
We are grateful for financial support from the National Natural Science Foundation of China (no. 21272191, 21302048 and 21572186), the Natural Science Foundation of Fujian Province for Distinguished Young Scholars (no. 2015J06003), the Fundamental Research Funds for the Central Universities (no. 20720150045), NFFTBS (no. J1310024) and the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT).
Notes and references
- For selected reviews, see: (a) A. Y. Khan and G. Suresh Kumar, Biophys. Rev., 2015, 7, 407 CrossRef CAS; (b) M. Iranshahy, R. J. Quinn and M. Iranshahi, RSC Adv., 2014, 4, 15900 RSC; (c) K. W. Bentley, Nat. Prod. Rep., 2006, 23, 444 RSC; (d) K. W. Bentley, Nat. Prod. Rep., 2005, 22, 249 RSC; (e) K. W. Bentley, Nat. Prod. Rep., 2004, 21, 395 RSC; (f) K. W. Bentley, in The Isoquinoline Alkaloids, Hardwood Academic, Amsterdam, 1998; vol. 1 Search PubMed; (g) J. D. Phillipson, M. F. Roberts and M. H. Zenk, The Chemistry and Biology of Isoquinoline Alkaloids, Springer Verlag, Berlin, 1985 Search PubMed.
- For recent selected reviews, see: (a) L. Ackermann, Acc. Chem. Res., 2014, 47, 281 CrossRef CAS PubMed; (b) R. He, Z.-T. Huang, Q.-Y. Zheng and C. Wang, Tetrahedron Lett., 2014, 55, 5705 CrossRef CAS; (c) G. Zeni and R. C. Larock, Chem. Rev., 2004, 104, 2285 CrossRef CAS PubMed; (d) I. Nakamura and Y. Yamamoto, Chem. Rev., 2004, 104, 2127 CrossRef CAS PubMed.
- Z. Huo and Y. Yamamoto, Tetrahedron Lett., 2009, 50, 3651 CrossRef CAS.
- Y.-N. Niu, Z.-Y. Yan, G.-L. Gao, H.-L. Wang, X.-Z. Shu, K.-G. Ji and Y.-M. Liang, J. Org. Chem., 2009, 74, 2893 CrossRef CAS PubMed.
- (a) Z. Huo, I. D. Gridnev and Y. Yamamoto, J. Org. Chem., 2010, 75, 1266 CrossRef CAS PubMed; (b) D. Fischer, H. Tomeba, N. K. Pahadi, N. T. Patil, Z. Huo and Y. Yamamoto, J. Am. Chem. Soc., 2008, 130, 15720 CrossRef CAS PubMed; (c) D. Fischer, H. Tomeba, N. K. Pahadi, N. T. Patil and Y. Yamamoto, Angew. Chem., Int. Ed., 2007, 46, 4764 CrossRef CAS PubMed.
- For the relevant palladium-catalyzed cyclization of 2-alkynyl benzyl azides, see: (a) J. Luo, Z. Huo, J. Fu, F. Jin and Y. Yamamoto, Org. Biomol. Chem., 2015, 13, 3227 RSC; (b) J. Luo, Z. Huo, J. Fu, F. Jin and Y. Yamamoto, Tetrahedron Lett., 2014, 55, 1552 CrossRef CAS; (c) H.-P. Zhang, X.-H. Yang, P. Peng and J.-H. Li, Synthesis, 2011, 1219 CAS; (d) H.-P. Zhang, S.-C. Yu, Y. Liang, P. Peng, B.-X. Tang and J.-H. Li, Synlett, 2011, 7, 982 Search PubMed.
- For recent reviews on ynamide reactivity, see: (a) X.-N. Wang, H.-S. Yeom, L.-C. Fang, S. He, Z.-X. Ma, B. L. Kedrowski and R. P. Hsung, Acc. Chem. Res., 2014, 47, 560 CrossRef CAS PubMed; (b) K. A. DeKorver, H. Li, A. G. Lohse, R. Hayashi, Z. Lu, Y. Zhang and R. P. Hsung, Chem. Rev., 2010, 110, 5064 CrossRef CAS PubMed; (c) G. Evano, A. Coste and K. Jouvin, Angew. Chem., Int. Ed., 2010, 49, 2840 CrossRef CAS PubMed.
- For our recent study on the transition metal-catalyzed oxidation-initiated tandem reactions based on ynamides, see: (a) L. Li, B. Zhou, Y.-H. Wang, C. Shu, Y.-F. Pan, X. Lu and L.-W. Ye, Angew. Chem., Int. Ed., 2015, 54, 8245 CrossRef CAS PubMed; (b) F. Pan, C. Shu, Y.-F. Ping, Y.-F. Pan, P.-P. Ruan, Q.-R. Fei and L.-W. Ye, J. Org. Chem., 2015, 80, 10009 CrossRef CAS PubMed; (c) L. Li, B. Zhou and L.-W. Ye, Chin. J. Org. Chem., 2015, 35, 655 CrossRef CAS; (d) L. Li, C. Shu, B. Zhou, Y.-F. Yu, X.-Y. Xiao and L.-W. Ye, Chem. Sci., 2014, 5, 4057 RSC; (e) F. Pan, S. Liu, C. Shu, R.-K. Lin, Y.-F. Yu, J.-M. Zhou and L.-W. Ye, Chem. Commun., 2014, 50, 10726 RSC; (f) C.-H. Shen, L. Li, W. Zhang, S. Liu, C. Shu, Y.-E. Xie, Y.-F. Yu and L.-W. Ye, J. Org. Chem., 2014, 79, 9313 CrossRef CAS PubMed.
- For our recent study on the Au-catalyzed amination-initiated tandem reactions based on ynamides, see: (a) C. Shu, Y.-H. Wang, B. Zhou, X.-L. Li, Y.-F. Ping, X. Lu and L.-W. Ye, J. Am. Chem. Soc., 2015, 137, 9567 CrossRef CAS PubMed; (b) A.-H. Zhou, Q. He, C. Shu, Y.-F. Yu, S. Liu, T. Zhao, W. Zhang, X. Lu and L.-W. Ye, Chem. Sci., 2015, 6, 1265 RSC; (c) X.-Y. Xiao, A.-H. Zhou, C. Shu, F. Pan, T. Li and L.-W. Ye, Chem. – Asian J., 2015, 10, 1854 CrossRef CAS PubMed; (d) C.-H. Shen, Y. Pan, Y.-F. Yu, Z.-S. Wang, W. He, T. Li and L.-W. Ye, J. Organomet. Chem., 2015, 795, 63 CrossRef CAS.
- For recent selected reviews on the generation of gold carbenes, see: (a) R. Dorel and A. M. Echavarren, Chem. Rev., 2015, 115, 9028 CrossRef CAS PubMed; (b) D. Qian and J. Zhang, Chem. Soc. Rev., 2015, 44, 677 RSC; (c) Y. Wang, M. E. Muratore and A. M. Echavarren, Chem. – Eur. J., 2015, 21, 7332 CrossRef CAS PubMed; (d) F. Wei, C. Song, Y. Ma, L. Zhou, C.-H. Tung and Z. Xu, Sci. Bull., 2015, 60, 1479 CrossRef CAS; (e) H.-S. Yeom and S. Shin, Acc. Chem. Res., 2014, 47, 966 CrossRef CAS PubMed; (f) L. Fensterbank and M. Malacria, Acc. Chem. Res., 2014, 47, 953 CrossRef CAS PubMed; (g) C. Obradors and A. M. Echavarren, Acc. Chem. Res., 2014, 47, 902 CrossRef CAS PubMed; (h) L. Zhang, Acc. Chem. Res., 2014, 47, 877 CrossRef CAS PubMed; (i) A. S. K. Hashmi, Acc. Chem. Res., 2014, 47, 864 CrossRef CAS PubMed; (j) C. Obradors and A. M. Echavarren, Chem. Commun., 2014, 50, 16 RSC; (k) J. Xiao and X. Li, Angew. Chem., Int. Ed., 2011, 50, 7226 CrossRef CAS PubMed; (l) A. S. K. Hashmi, Angew. Chem., Int. Ed., 2010, 49, 5232 CrossRef CAS PubMed.
- For recent selected examples on the generation of α-imino gold carbenes, see: (a) H. Jin, L. Huang, J. Xie, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2016, 55, 794 CrossRef CAS PubMed; (b) L. Zhu, Y. Yu, Z. Mao and X. Huang, Org. Lett., 2015, 17, 30 CrossRef CAS PubMed; (c) S. K. Pawar, R. L. Sahani and R.-S. Liu, Chem. – Eur. J., 2015, 21, 10843 CrossRef CAS PubMed; (d) A. Prechter, G. Henrion, P. F. dit Bel and F. Gagosz, Angew. Chem., Int. Ed., 2014, 53, 4959 CrossRef CAS PubMed; (e) M. Garzón and P. W. Davies, Org. Lett., 2014, 16, 4850 CrossRef PubMed; (f) Y. Tokimizu, S. Oishi, N. Fujii and H. Ohno, Org. Lett., 2014, 16, 3138 CrossRef CAS PubMed; (g) E. Chatzopoulou and P. W. Davies, Chem. Commun., 2013, 49, 8617 RSC; (h) Z.-Y. Yan, Y. Xiao and L. Zhang, Angew. Chem., Int. Ed., 2012, 51, 8624 CrossRef CAS PubMed; (i) Y. Xiao and L. Zhang, Org. Lett., 2012, 14, 4662 CrossRef CAS PubMed; (j) P. W. Davies, A. Cremonesi and L. Dumitrescu, Angew. Chem., Int. Ed., 2011, 50, 8931 CrossRef CAS PubMed; (k) B. Lu, Y. Luo, L. Liu, L. Ye, Y. Wang and L. Zhang, Angew. Chem., Int. Ed., 2011, 50, 8358 CrossRef CAS PubMed; (l) A. Wetzel and F. Gagosz, Angew. Chem., Int. Ed., 2011, 50, 7354 CrossRef CAS PubMed; (m) C. Li and L. Zhang, Org. Lett., 2011, 13, 1738 CrossRef CAS PubMed; (n) D. J. Gorin, N. R. Davis and F. D. Toste, J. Am. Chem. Soc., 2005, 127, 11260 CrossRef CAS PubMed.
- For recent selected examples, see: (a) E. Arbačiauskienė, V. Laukaitytė, W. Holzer and A. Šačkus, Eur. J. Org. Chem., 2015, 5663 CrossRef; (b) R. Jeyachandran, H. K. Potukuchi and L. Ackermann, Beilstein J. Org. Chem., 2012, 8, 1771 CrossRef CAS PubMed; (c) S. Chandrasekhar, M. Seenaiah, A. Kumar, C. R. Reddy, S. K. Mamidyala, G. Chityal and S. Balasubramanian, Tetrahedron Lett., 2011, 52, 806 CrossRef CAS.
- N. Sin, B. L. Venables, L.-Q. Sun, S.-Y. Sit, Y. Chen and P. M. Scola, PCT Int. Appl. WO2008060927A2, 2008 Search PubMed.
- Attempts to trap this kind of α-imino gold carbene intermolecularly by using different types of external alcohols under various reaction conditions only led to the formation of background 3-aminoisoquinoline.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qo00033a |
| This journal is © the Partner Organisations 2016 |