Au/TiO2 catalyzed reductive amination of aldehydes and ketones using formic acid as reductant†
Shengzong
Liang
a,
Paige
Monsen
a,
Gerald B.
Hammond
*a and
Bo
Xu
*b
aDepartment of Chemistry, University of Louisville, Louisville, Kentucky 40292, USA. E-mail: gb.hammond@louisville.edu
bCollege of Chemistry, Chemical Engineering and Biotechnology, Donghua University, 2999 North Renmin Lu, Shanghai 201620, China. E-mail: bo.xu@dhu.edu.cn
First published on 18th February 2016
Abstract
The combination of commercial easily available Au/TiO2 as catalyst and cost-effective formic acid as reductant was able to render reductive amination of various carbonyl compounds. Aldehydes and ketones were converted into secondary and tertiary amines in good to excellent yields. The supported gold nanoparticles could be easily recycled by simple filtration.
The carbon–nitrogen bond formation is one of the most important transformations in organic chemistry because numerous biologically active compounds, dyes, agrochemicals and functionalized materials contain nitrogen.1 Various methodologies have been established for the construction of nitrogen-containing compounds.2 Among these methods, reductive amination of aldehydes and ketones is efficient and straightforward.3 Conventional reductive amination protocols rely for the most part on stoichiometric amounts of boron-,4 tin-5 and silane-6 based reductants, but they are costly and usually give rise to over-alkylation or the formation of toxic byproducts. Some reductive amination methodologies were also reported by using gas reductant such as CO and H2, but mostly very harsh condition (high pressure and temperature) was needed.7 Moreover, few reductive amination of secondary amines in purpose of tertiary amine synthesis was reported because of the steric hindrance for the both formation and reduction of iminium/enamine.8 Reductive amination has also been used in chiral amine synthesis, albeit the unstable imine intermediates may need isolation.9 Formic acid has been widely used as reductant in various transfer hydrogen strategies because it is inexpensive and easy to handle. In addition, the by-product is CO2 gas that can be easily recycled, thus offering a more eco-friendly reaction conditions.10 Therefore, formic acid is a good alternative transfer hydrogen reagent for reductive amination. However, such formic acid-based reductive amination was rarely reported. Furthermore, in the few reported examples, the specially designed, costly, and non-recyclable homogeneous transition metal catalysts such as Ru and Ir were usually used.11 The main text of the article should appear here with headings as appropriate.
Supported nanoparticle catalysts have attracted much interest in organic synthesis due to their high activity, recyclability and good stability properties.12 Notably, supported gold nanoparticles (Au/NPs) were able to catalyze many reactions such as oxidation,13 reduction,2h,14 hydrosilylation,15 cross-coupling,16 as well as π bond activation.17 Although Au/NPs showed high efficiency in hydride transfer process, allowing the success of N-alkylation of amines using alcohols through a “borrowing hydrogen strategy”,18 the use of Au/NPs for amine synthesis through reductive amination strategy is rare.19 In addition, the N-alkylation of amines catalyzed by Au/NPs through borrowing hydrogen strategy usually needed high pressure and temperature and also only primary amines could be used as substrates, so these drawbacks limited its applications in construction of tertiary amines. Herein, in this work, we reported the reductive amination of aldehydes and ketones catalyzed by commercial Au/TiO2via introduction of external hydrogen source – inexpensive and environmentally benign formic acid. A wide range of secondary and tertiary amines were obtained. Moreover, this gold catalyst could be easily recycled without significant loss of reactivity.
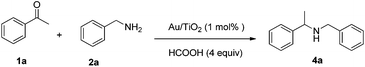
Initially, we chose reductive amination of acetophenone 1a and benzylamine 2a as our model reaction (Table 1). Different solvents were first screened; the reaction in t-BuOH provided the best yield (68%) when Au/TiO2 (1 mol%), 2a (2 equiv.) and HCOOH (4 equiv.) were used at 80 °C (Table 1, entries 1–6). However, it was found that the rest of benzylamine was consumed by formic acid to form N-benzylformamide as byproduct, which inhibited the reaction. An attempt to lower the temperature (60 °C) failed to increase the yield (Table 1, entry 7), but when more benzylamine was used as “sacrifice” (4 equiv.) the desired N-benzyl-1-phenylethanamin was obtained in high yield (91%) (Table 1, entry 8). Increasing the reaction temperature and reducing the amount of formic acid led to reduced yields (Table 1, entries 9 and 10). Au/TiO2 and HCOOH were indispensable for this reductive amination (Table 1, entries 11 and 12). Moreover, nanogold particles with another support (Al2O3) was tested, but the efficiency was almost the same as with Au/TiO2; this result proved that the support did not play an important role in this reductive amination process (Table 1, entry 13).
| Entry | Solvent |
1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a 2a |
T (°C) | t (h) | Yielda (%) |
|---|---|---|---|---|---|
| a Yields were determined by 1H NMR using 1,3,5-trimethoxybenzene as internal standard. b HCOOH (2 equiv.) is used. c No Au/TiO2 was used. d No HCOOH was used. e Au/Al2O3 (1 mol%) was used. | |||||
| 1 | Dioxane | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 46 |
| 2 | EtOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 62 |
| 3 | DMF | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 50 |
| 4 | t-BuOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 68 |
| 5 | Tol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 56 |
| 6 | THF | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 42 |
| 7 | t-BuOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
60 | 22 | 63 |
| 8 | t-BuOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
60 | 22 | 91 |
| 9 | t-BuOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
70 | 22 | 77 |
| 10b | t-BuOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
60 | 22 | 63 |
| 11c | t-BuOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
60 | 22 | 0 |
| 12d | t-BuOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
60 | 22 | 0 |
| 13e | t-BuOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
60 | 22 | 90 |
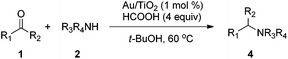
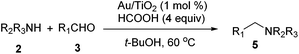
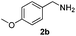
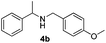
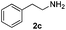
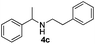
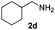
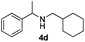
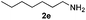
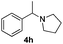
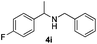
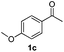
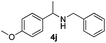
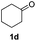
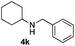
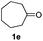
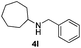
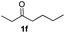
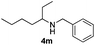
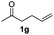
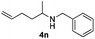
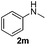
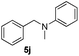
With the optimized protocol in hand, the Au/TiO2 catalyzed reductive amination of ketone substrates were explored (Table 2). First, the reductive amination of acetophenone 1a with different primary amines was tested, and good to excellent yields were obtained for all these primary amines (Table 2, entries 1–7). Pyrrolidine also worked well to afford corresponding 1-(1-phenylethyl)pyrrolidine 4h with excellent yield (95%, Table 2, entry 8). Both electron withdrawing and donating groups on acetophenone didn't affect the activity (Table 2, entries 9 and 10) and both cyclic and noncyclic aliphatic ketones gave the corresponding amine products in high yields (Table 2, entries 11–14). Moreover, a variety of aldehyde substrates were also tested and most of them could be converted into the corresponding amine products in shorter time than ketones (Table 3). Primary amines (Table 3, entries 1–5), cyclic secondary amines (Table 3, entries 6–8) and acyclic secondary amines (Table 3, entries 9 and 10) worked very well when reacted with benzaldehyde 3a. Less basic amines like N-methylaniline 2m needed longer reaction times (Table 3, entry 10). Electron deficient aldehydes could speed up the reaction with N-methylaniline to some extent due to the enhanced electron-deficiency on the carbonyl carbon (Table 3, entries 11 and 12). Furthermore, a variety of substituted benzaldehydes (Table 3, entries 13–16), 1-naphthaldehyde (Table 3, entry 17), and cyclohexanecarbaldehyde (Table 3, entries 18 and 19) were also examined and in all cases excellent reactivity were observed.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | 1 | 2 | 4 | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1 (0.25 mmol), 2 (1 mmol), Au/TiO2 (1 mol%), HCOOH (1 mmol) in t-BuOH (0.25 ml) at 60 °C. b Isolated yields. c Au/TiO2 (2 mol%) was used at 70 °C. | |||||
| 1 |
|
|
|
22 | 88 |
| 2 | 1a |
|
|
24 | 86 |
| 3c | 1a |
|
|
24 | 90 |
| 4c | 1a |
|
|
18 | 94 |
| 5c | 1a |
|
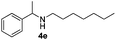
|
24 | 89 |
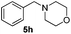
| 6c | 1a |
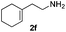
|
|
24 | 93 |
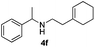
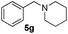
| 7c | 1a |
|
|
24 | 92 |
| 8c | 1a |
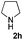
|
|
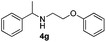
25 | 85 |
| 9 |
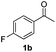
|
2a |
|
27 | 93 |
| 10 |
|
2a |
|
25 | 97 |
| 11c |
|
2a |
|
5 | 97 |
| 12c |
|
2a |
|
6 | 98 |
| 13c |
|
2a |
|
6 | 96 |
| 14c |
|
2a |
|
6 | 93 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
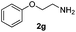
| Entry | 2 | 3 | 5 | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 3 (0.25 mmol), 2 (1 mmol), Au/TiO2 (1 mol%), HCOOH (1 mmol) in t-BuOH (0.25 mL) at 60 °C. b Isolated yields. | |||||
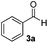
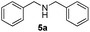
| 1 |
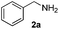
|
|
|
3 | 98 |
| 2 |
|
3a |
|
3 | 97 |
| 3 |
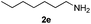
|
3a |
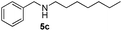
|
6 | 94 |
| 4 |
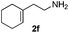
|
3a |
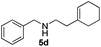
|
6 | 93 |
| 5 |
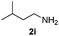
|
3a |
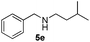
|
5 | 92 |
| 6 |
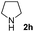
|
3a |
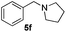
|
4 | 98 |
| 7 |
|
3a |
|
4 | 99 |
| 8 |
|
3a |
|
4 | 98 |
| 9 |
|
3a |
|
4 | 96 |
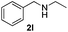
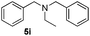
| 10 |
|
3a |
|
24 | 95 |
| 11 | 2m |
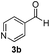
|
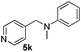
|
5 | 84 |
| 12 | 2m |
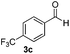
|
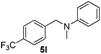
|
12 | 94 |
| 13 | 2m |
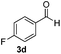
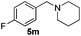
|
|
4 | 99 |
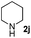
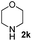
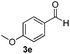
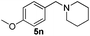
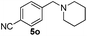
| 14 | 2j |
|
|
6 | 98 |
| 15 | 2j |
|
|
4 | 98 |
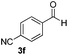
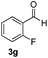
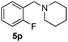
| 16 | 2j |
|
|
4 | 96 |
| 17 | 2j |
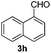
|
|
4 | 96 |
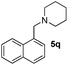
| 18 | 2a |
|
5b | 5 | 98 |
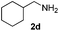
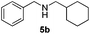
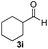
| 19 | 2d | 3i |
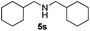
|
8 | 98 |
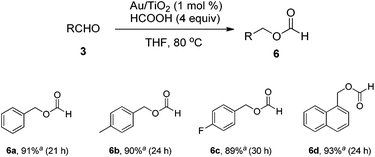
It was also found that when the amine partners were absent, the tandem reduction/formylation product 6 could be obtained in one pot. Because deformylation could be achieved selectively in the presence of other ester groups, this O-formylation method may prove beneficial on those reactions that need to protect alcohol groups in a complex synthetic sequence. Meanwhile, the O-formylation products also testified the excellent activity for hydride transfer of Au/NPs. Three substituted benzaldehydes and 1-naphthaldehyde were tested using THF as solvent, all of them provided the desired formates 6 in good yields (Scheme 1).
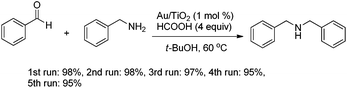
To take advantage of the recyclability of this heterogeneous catalyst, five straight run of reductive amination were conducted (Scheme 2). After each run, Au/TiO2 was recovered by simple filtration. High activity of Au/TiO2 was retained after five runs with only slightly decreased yields observed. The slow deactivation of gold catalyst was caused by the aggregation of gold nanoparticles, which has been demonstrated by our previous study (Scheme 3).17e
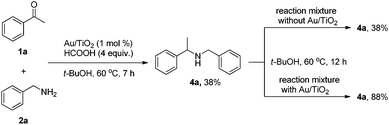
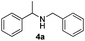
Furthermore, in order to clarify whether catalytic gold species leached out from the TiO2 support into the reaction mixture or not, a leaching experiment was carried out. The reductive amination of acetophenone 1a with benzylamine 2a was conducted following the standard condition. After 7 hours 4a was formed in the yield of 38%, and meanwhile the reaction mixture (150 μl) was transferred to the other reaction vial. Both the residue with Au/TiO2 and the reaction mixture without Au/TiO2 were then heated for another 12 hours. It was found that the residual containing Au/TiO2 catalyst further produced 4a with 88% yield, in contrast, in the absence of Au/TiO2 the reaction stopped with unchanged 38% yield of 4a. These results indicated that the reductive amination was not catalyzed by leaching catalytic gold species.
In summary, we have developed an efficient reductive amination methodology, in which commercial available and easily recyclable heterogeneous Au/TiO2 was used as catalyst, and also cost-effective and environmentally friendly formic acid was used as transfer hydrogen reagent. This combination allowed the formation of various amines from aldehydes and ketones with good reactivity. Our method has a potential of being a good complement for conventional reductive amination.
Acknowledgements
We are grateful to the National Science Foundation for financial support (CHE-1401700). BX is grateful to the National Science Foundation of China for financial support (NSFC-21472018).References
-
(a) J. F. Hartwig, Acc. Chem. Res., 2008, 41, 1534–1544 CrossRef CAS PubMed
; (b) G. Guillena, D. J. Ramón and M. Yus, Chem. Rev., 2010, 110, 1611–1641 CrossRef CAS PubMed
; (c) A. A. N. Magro, G. R. Eastham and D. J. Cole-Hamilton, Chem. Commun., 2007, 3154–3156 RSC
; (d) T. E. Müller, K. C. Hultzsch, M. Yus, F. Foubelo and M. Tada, Chem. Rev., 2008, 108, 3795–3892 CrossRef PubMed
.
-
(a) Y. Luo, K. Ji, Y. Li and L. Zhang, J. Am. Chem. Soc., 2012, 134, 17412–17415 CrossRef CAS PubMed
; (b) L.-C. Lee and Y. Zhao, ACS Catal., 2014, 4, 688–691 CrossRef CAS
; (c) H. Duan, S. Sengupta, J. L. Petersen, N. G. Akhmedov and X. Shi, J. Am. Chem. Soc., 2009, 131, 12100–12102 CrossRef CAS PubMed
; (d) M. H. S. A. Hamid, P. A. Slatford and J. M. J. Williams, Adv. Synth. Catal., 2007, 349, 1555–1575 CrossRef CAS
; (e) D. M. T. Chan, K. L. Monaco, R. Li, D. Bonne, C. G. Clark and P. Y. S. Lam, Tetrahedron Lett., 2003, 44, 3863–3865 CrossRef CAS
; (f) G. D. Vo and J. F. Hartwig, J. Am. Chem. Soc., 2009, 131, 11049–11061 CrossRef CAS PubMed
; (g) B. P. Fors and S. L. Buchwald, J. Am. Chem. Soc., 2010, 132, 15914–15917 CrossRef CAS PubMed
; (h) A. Corma and P. Serna, Science, 2006, 313, 332–334 CrossRef CAS PubMed
.
-
(a) A. F. Abdel-Magid and S. J. Mehrman, Org. Process Res. Dev., 2006, 10, 971–1031 CrossRef CAS
; (b) T. Gross, A. M. Seayad, M. Ahmad and M. Beller, Org. Lett., 2002, 4, 2055–2058 CrossRef CAS PubMed
; (c) M. Zhang, H. Yang, Y. Zhang, C. Zhu, W. Li, Y. Cheng and H. Hu, Chem. Commun., 2011, 47, 6605–6607 RSC
; (d) D. Talwar, N. P. Salguero, C. M. Robertson and J. Xiao, Chem. – Eur. J., 2014, 20, 245–252 CrossRef CAS PubMed
; (e) S. Werkmeister, K. Junge and M. Beller, Green Chem., 2012, 14, 2371–2374 RSC
; (f) D. Menche, J. Hassfeld, J. Li, G. Menche, A. Ritter and S. Rudolph, Org. Lett., 2006, 8, 741–744 CrossRef CAS PubMed
; (g) K. Saito and T. Akiyama, Chem. Commun., 2012, 48, 4573–4575 RSC
.
-
(a) M. Horn, H. Mayr, E. Lacôte, E. Merling, J. Deaner, S. Wells, T. McFadden and D. P. Curran, Org. Lett., 2012, 14, 82–85 CrossRef CAS PubMed
; (b) W. Liao, Y. Chen, Y. Liu, H. Duan, J. L. Petersen and X. Shi, Chem. Commun., 2009, 6436–6438 RSC
; (c) E. M. Dangerfield, C. H. Plunkett, A. L. Win-Mason, B. L. Stocker and M. S. M. Timmer, J. Org. Chem., 2010, 75, 5470–5477 CrossRef CAS PubMed
.
-
(a) P. D. Pham, P. Bertus and S. Legoupy, Chem. Commun., 2009, 6207–6209 RSC
; (b) H. Kato, I. Shibata, Y. Yasaka, S. Tsunoi, M. Yasuda and A. Baba, Chem. Commun., 2006, 4189–4191 RSC
.
-
(a) O.-Y. Lee, K.-L. Law, C.-Y. Ho and D. Yang, J. Org. Chem., 2008, 73, 8829–8837 CrossRef CAS PubMed
; (b) T. Mizuta, S. Sakaguchi and Y. Ishii, J. Org. Chem., 2005, 70, 2195–2199 CrossRef CAS PubMed
; (c) O.-Y. Lee, K.-L. Law and D. Yang, Org. Lett., 2009, 11, 3302–3305 CrossRef CAS PubMed
.
-
(a) J. W. Park and Y. K. Chung, ACS Catal., 2015, 5, 4846–4850 CrossRef CAS
; (b) D. Chusov and B. List, Angew. Chem., Int. Ed., 2014, 53, 5199–5201 CAS
; (c) M. Chang, S. Liu, K. Huang and X. Zhang, Org. Lett., 2013, 15, 4354–4357 CrossRef CAS PubMed
; (d) A. Pagnoux-Ozherelyeva, N. Pannetier, M. D. Mbaye, S. Gaillard and J.-L. Renaud, Angew. Chem., Int. Ed., 2012, 51, 4976–4980 CrossRef CAS PubMed
; (e) S. Fleischer, S. Zhou, K. Junge and M. Beller, Chem. – Asian. J., 2011, 6, 2240–2245 CrossRef CAS PubMed
; (f) D. B. Bagal, R. A. Watile, M. V. Khedkar, K. P. Dhake and B. M. Bhanage, Catal. Sci. Technol., 2012, 2, 354–358 RSC
; (g) A. S. Touchy, S. M. A. Hakim Siddiki, K. Kon and K.-i. Shimizu, ACS Catal., 2014, 4, 3045–3050 CrossRef CAS
.
- O. S. Nayal, V. Bhatt, S. Sharma and N. Kumar, J. Org. Chem., 2015, 80, 5912–5918 CrossRef CAS PubMed
.
-
(a) A. Bartoszewicz, N. Ahlsten and B. Martín-Matute, Chem. – Eur. J., 2013, 19, 7274–7302 CrossRef CAS PubMed
; (b) K. H. Hopmann and A. Bayer, Coord. Chem. Rev., 2014, 268, 59–82 CrossRef CAS
; (c) E. Menéndez-Pedregal, M. Vaquero, E. Lastra, P. Gamasa and A. Pizzano, Chem. – Eur. J., 2015, 21, 549–553 CrossRef PubMed
; (d) H. Xu, P. Yang, P. Chuanprasit, H. Hirao and J. Zhou, Angew. Chem., Int. Ed., 2015, 54, 5112–5116 CrossRef CAS PubMed
; (e) V. I. Tararov and A. Börner, Synlett, 2005, 203–211 CrossRef CAS
.
-
(a) D. Zhang, F. Ye, T. Xue, Y. Guan and Y. M. Wang, Catal. Today, 2014, 234, 133–138 CrossRef CAS
; (b) S. Ahammed, A. Saha and B. C. Ranu, J. Org. Chem., 2011, 76, 7235–7239 CrossRef CAS PubMed
.
-
(a) Q. Lei, Y. Wei, D. Talwar, C. Wang, D. Xue and J. Xiao, Chem. – Eur. J., 2013, 19, 4021–4029 CrossRef CAS PubMed
; (b) C. Wang, A. Pettman, J. Bacsa and J. Xiao, Angew. Chem., Int. Ed., 2010, 49, 7548–7552 CrossRef CAS PubMed
; (c) D. Gülcemal, S. Gülcemal, C. M. Robertson and J. Xiao, Organometallics, 2015, 34, 4394–4400 CrossRef
; (d) Z. Sahli, B. Sundararaju, M. Achard and C. Bruneau, Org. Lett., 2011, 13, 3964–3967 CrossRef CAS PubMed
.
-
(a) C. A. Witham, W. Huang, C.-K. Tsung, J. N. Kuhn, G. A. Somorjai and F. D. Toste, Nat. Chem., 2010, 2, 36–41 CrossRef CAS PubMed
; (b) D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852–7872 CrossRef CAS PubMed
; (c) H. Cong and J. A. Porco, ACS Catal., 2012, 2, 65–70 CrossRef CAS PubMed
; (d) A. Corma and H. Garcia, Chem. Soc. Rev., 2008, 37, 2096–2126 RSC
; (e) C. Qi, T. Qin, D. Suzuki and J. A. Porco, J. Am. Chem. Soc., 2014, 136, 3374–3377 CrossRef CAS PubMed
; (f) C. Qi, H. Cong, K. J. Cahill, P. Müller, R. P. Johnson and J. A. Porco, Angew. Chem., Int. Ed., 2013, 52, 8345–8348 CrossRef CAS PubMed
; (g) Y. Zhang, X. Cui, F. Shi and Y. Deng, Chem. Rev., 2012, 112, 2467–2505 CrossRef CAS PubMed
; (h) M. Stratakis and H. Garcia, Chem. Rev., 2012, 112, 4469–4506 CrossRef CAS PubMed
; (i) X. Liu, L. He, Y.-M. Liu and Y. Cao, Acc. Chem. Res., 2014, 47, 793–804 CrossRef CAS PubMed
.
-
(a) J. Restrepo, R. Porcar, P. Lozano, M. I. Burguete, E. García-Verdugo and S. V. Luis, ACS Catal., 2015, 5, 4743–4750 CrossRef CAS
; (b) S. Yudha, I. Kusuma and N. Asao, Tetrahedron, 2015, 71, 6459–6462 CrossRef
.
-
(a) Y. S. Wagh and N. Asao, J. Org. Chem., 2015, 80, 847–851 CrossRef CAS PubMed
; (b) F.-Z. Su, L. He, J. Ni, Y. Cao, H.-Y. He and K.-N. Fan, Chem. Commun., 2008, 3531–3533 RSC
; (c) L. He, L.-C. Wang, H. Sun, J. Ni, Y. Cao, H.-Y. He and K.-N. Fan, Angew. Chem., Int. Ed., 2009, 48, 9538–9541 CrossRef CAS PubMed
; (d) E. Vasilikogiannaki, I. Titilas, G. Vassilikogiannakis and M. Stratakis, Chem. Commun., 2015, 51, 2384–2387 RSC
.
-
(a) I. N. Lykakis, A. Psyllaki and M. Stratakis, J. Am. Chem. Soc., 2011, 133, 10426–10429 CrossRef CAS PubMed
; (b) I. Titilas, M. Kidonakis, C. Gryparis and M. Stratakis, Organometallics, 2015, 34, 1597–1600 CrossRef CAS
.
-
(a) J. Han, Y. Liu and R. Guo, J. Am. Chem. Soc., 2009, 131, 2060–2061 CrossRef CAS PubMed
; (b) S. Carrettin, J. Guzman and A. Corma, Angew. Chem., Int. Ed., 2005, 44, 2242–2245 CrossRef CAS PubMed
; (c) A. Moragues, F. Neaţu, V. I. Pârvulescu, M. D. Marcos, P. Amorós and V. Michelet, ACS Catal., 2015, 5, 5060–5067 CrossRef CAS
.
-
(a) X. Zhang and A. Corma, Angew. Chem., Int. Ed., 2008, 47, 4358–4361 CrossRef CAS PubMed
; (b) G. Villaverde, A. Corma, M. Iglesias and F. Sánchez, ACS Catal., 2012, 2, 399–406 CrossRef CAS
; (c) L. Tang, X. Guo, Y. Yang, Z. Zha and Z. Wang, Chem. Commun., 2014, 50, 6145–6148 RSC
; (d) C. Efe, I. N. Lykakis and M. Stratakis, Chem. Commun., 2011, 47, 803–805 RSC
; (e) S. Liang, J. Jasinski, G. B. Hammond and B. Xu, Org. Lett., 2015, 17, 162–165 CrossRef CAS PubMed
.
-
(a) L. He, X.-B. Lou, J. Ni, Y.-M. Liu, Y. Cao, H.-Y. He and K.-N. Fan, Chem. – Eur. J., 2010, 16, 13965–13969 CrossRef CAS PubMed
; (b) C.-H. Tang, L. He, Y.-M. Liu, Y. Cao, H.-Y. He and K.-N. Fan, Chem. – Eur. J., 2011, 17, 7172–7177 CrossRef CAS PubMed
; (c) L. Tang, Y. Yang, L. Wen, S. Zhang, Z. Zha and Z. Wang, Org. Chem. Front., 2015, 2, 114–118 RSC
.
- R. Kumar, E. Gravel, A. Hagège, H. Li, D. Verma, I. N. N. Namboothiri and E. Doris, ChemCatChem, 2013, 5, 3571–3575 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental, spectral data and copies of spectra. See DOI: 10.1039/c5qo00439j |
| This journal is © the Partner Organisations 2016 |