DOI:
10.1039/C5QO00430F
(Research Article)
Org. Chem. Front., 2016,
3, 385-393
Metal-free oxidative hydrophosphinylation of 1,7-enynes†
Received
17th December 2015
, Accepted 22nd January 2016
First published on 25th January 2016
Abstract
A new metal-free oxidative hydrophosphinylation of 1,7-enynes for forming polyfunctionalized 3,4-dihydroquinolin-2(1H)-ones has been realized using readily accessible diarylphosphine oxide and TBPB as an oxidant. The reaction pathway involves an in situ-generated P-centered radical-triggered α,β-conjugate addition/6-exo-dig cyclization/H-abstraction sequence, providing a direct and promising protocol for the formation of C–P, C–C and C–H bonds and rapid construction of complex heterocyclic compounds.
Introduction
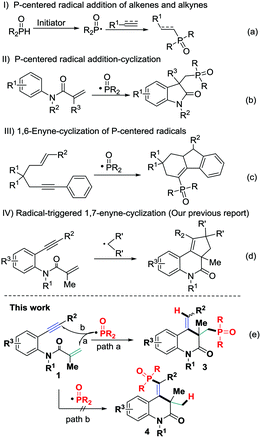
Organophosphorus compounds can be found to have a wide range of applications in organic synthesis,1 medicinal chemistry,2 and materials science.3 They have also served as excellent ligands for transition-metal catalysis and organocatalysts.4 In view of their significance, many efforts have been devoted to paving the pathways to a collection of phosphorus compounds, which has made the methods more powerful and applicable. Basically, two general strategies can be summarized: traditional transition-metal catalyzed coupling reactions5 and alkene or alkyne functionalization of suitable phosphorus precursors.6 The latter provides direct and atom economic approaches for forming organophosphorus compounds with good functional group compatibility, especially in the recently well-developed addition of P-centered radicals to unsaturated systems7 (Scheme 1a and b), and thus represents a highly desirable methodology.8
 |
| | Scheme 1 Profiles for the radical synthesis of phosphorus compounds. | |
1,n-enynes serve as important and useful building blocks for substrate-specific cyclization cascades and have been extensively utilized in organic syntheses.9 They can readily result in compounds with multiple functionalities via synergistic processes across the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond systems in a single step fashion. Specifically, oxidative radical 1,n-enyne-cyclizations have gradually become a powerful tool for rapidly constructing cyclic compounds.10 These reactions not only feature high atom-economy, annulation efficiency, and excellent functional group compatibility but also avoid prefunctionalization of the reaction substrates or the use of expensive transition metals. Recently, our groups11 and others12 have developed a series of unique radical 1,n-enyne-cyclizations for the construction of functionalized complex molecules of chemical and pharmaceutical interest. For instance, Liang and coworkers reported a phosphorus radical-involved 1,6-enyne-cyclization reaction to synthesize fluorene derivatives (Scheme 1c).12h However, in sharp contrast, phosphorus radical triggered 1,7-enyne-cyclizations are virtually unexplored partly because of the poor selectivity of the radical addition toward alkenyl (Scheme 1e, path a) and alkynyl (Scheme 1e, path b) groups. Thus, regioselective radical addition-cyclization of 1,7-enynes is highly challenging. Very recently, we developed the addition of various C-centered radicals to N-tethered 1,7-enynes 1, which underwent a radical addition-cyclization/H-abstraction/radical coupling sequence to give access to spirosubstituted cyclopenta[c]quinolones (Scheme 1d).11a We envisioned that, under the right set of oxidative conditions, phosphorus radicals could be preferentially engaged in alkenyl addition with the N-tethered 1,7-enynes 1 to control the reaction selectivity (Scheme 1e, path a), and thereby afford functionalized 3,4-dihydroquinolin-2(1H)-ones. Herein we report the successful implementation of this concept with a new cascade phosphorus-radical triggered 1,7-enyne-cyclization, which enabled the formation of a variety of phosphorus-containing 3,4-dihydroquinolin-2(1H)-ones through hydrophosphinylation of 1,7-enynes in a highly controlled manner (Scheme 1e).
C bond systems in a single step fashion. Specifically, oxidative radical 1,n-enyne-cyclizations have gradually become a powerful tool for rapidly constructing cyclic compounds.10 These reactions not only feature high atom-economy, annulation efficiency, and excellent functional group compatibility but also avoid prefunctionalization of the reaction substrates or the use of expensive transition metals. Recently, our groups11 and others12 have developed a series of unique radical 1,n-enyne-cyclizations for the construction of functionalized complex molecules of chemical and pharmaceutical interest. For instance, Liang and coworkers reported a phosphorus radical-involved 1,6-enyne-cyclization reaction to synthesize fluorene derivatives (Scheme 1c).12h However, in sharp contrast, phosphorus radical triggered 1,7-enyne-cyclizations are virtually unexplored partly because of the poor selectivity of the radical addition toward alkenyl (Scheme 1e, path a) and alkynyl (Scheme 1e, path b) groups. Thus, regioselective radical addition-cyclization of 1,7-enynes is highly challenging. Very recently, we developed the addition of various C-centered radicals to N-tethered 1,7-enynes 1, which underwent a radical addition-cyclization/H-abstraction/radical coupling sequence to give access to spirosubstituted cyclopenta[c]quinolones (Scheme 1d).11a We envisioned that, under the right set of oxidative conditions, phosphorus radicals could be preferentially engaged in alkenyl addition with the N-tethered 1,7-enynes 1 to control the reaction selectivity (Scheme 1e, path a), and thereby afford functionalized 3,4-dihydroquinolin-2(1H)-ones. Herein we report the successful implementation of this concept with a new cascade phosphorus-radical triggered 1,7-enyne-cyclization, which enabled the formation of a variety of phosphorus-containing 3,4-dihydroquinolin-2(1H)-ones through hydrophosphinylation of 1,7-enynes in a highly controlled manner (Scheme 1e).
Results and discussion
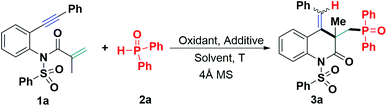
Initially, 1,7-enyne 1a and diphenylphosphine oxide (DPPO, 2a) were chosen as model substrates to test the possibility of an intermolecular radical addition-cyclization toward 3,4-dihydroquinoline formation, with different silver salts as the catalysts, acetonitrile (CH3CN) as the solvent and 4 Å molecular sieves (MS). The results are summarized in Table 1. In the presence of silver nitrate, the reaction proceeded to give the expected 3,4-dihydroquinolin-2(1H)-one 3a, albeit with a low yield (39%). Other silver salts like silver acetate and silver carbonate had a lower catalytic activity, resulting in unsatisfactory outcomes (entries 2 and 3). Increasing the loading of silver nitrate to 1.0 or 2.0 equivalents was harmful to the yield of 3a (entries 4 and 5). Next, we attempted to use organic oxidants to improve the reaction conditions. This screening of organic oxidants (2.0 equivalents) revealed that compared with tert-butyl hydroperoxide (TBHP, 70% in water), di-tert-butyl peroxide (DTBP) and benzoyl peroxide (BPO) (entries 6–8), tert-butyl peroxybenzoate (TBPB) gave the best result in this radical addition-cyclization, providing a 44% yield of 3a (entry 9). Besides, the reaction efficiency shows an important dependency on solvent. For example, the reaction in 1,4-dioxane worked more efficiently and delivered 3a in 49% yield whereas other aprotic solvents, such as dimethyl sulfoxide (DMSO), N,N-dimethyl formamide (DMF), ethyl acetate, toluene and 1,2-dichloroethane (DCE), proved to be far less effective than 1,4-dioxane (entry 10 vs. entries 11–15). Moreover, using a combination of TBPB with TBAI as the catalyst, nearly no product 3a was detected (entry 16), thus suggesting that the use of an ammonium salt suppressed the reaction process. It was also found that the reaction temperature had an important influence on the reaction efficiency. A lower conversion was observed with the reaction temperature at either 50 °C or 80 °C (entry 10 vs. entries 17 and 18). Other endeavors to improve the reaction efficiency were attempted as well, such as changing the amount of DPPO (3.0 or 4.0 equiv.) or TBPB (1.5 equiv.) (entries 19–21). To our delight, adjusting the amount of TBPB to 1.5 equivalents and simultaneously changing the substrate ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (1a
4 (1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a) using 1,4-dioxane at 60 °C further facilitated the reaction process and improved the yield of 3a to 61% (entry 21).
2a) using 1,4-dioxane at 60 °C further facilitated the reaction process and improved the yield of 3a to 61% (entry 21).
Table 1 Optimization of the reaction conditionsa
|
|
| |
Catalyst/oxidant (equiv.) |
Solvent |
T (°C) |
Yieldb (%) |
Reaction conditions: 1a (0.3 mmol), 2a (0.6 mmol), catalyst/oxidant, dry solvent (2.0 mL), and 4 Å MS (200 mg) for 6.0 hours.
Isolated yield of product 3a based on 1,7-enyne 1 using column chromatography.
These reactions were conducted under argon; the other reactions were conducted under air.
Using TBAI (10 mol%) as a catalyst; ND = not detected.
The ratio of 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a was 1 2a was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.
The ratio of 1a 3.
The ratio of 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a was 1 2a was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. 4.
|
| 1c |
AgNO3 (0.2) |
CH3CN |
60 |
39 |
| 2c |
AgOAc (0.2) |
CH3CN |
60 |
21 |
| 3c |
Ag2CO3 (0.2) |
CH3CN |
60 |
30 |
| 4c |
AgNO3 (1.0) |
CH3CN |
60 |
38 |
| 5c |
AgNO3 (2.0) |
CH3CN |
60 |
34 |
| 6 |
TBHP (2.0) |
CH3CN |
60 |
26 |
| 7 |
DTBP (2.0) |
CH3CN |
60 |
34 |
| 8 |
BPO (2.0) |
CH3CN |
60 |
31 |
| 9 |
TBPB (2.0) |
CH3CN |
60 |
44 |
| 10 |
TBPB (2.0) |
1,4-Dioxane |
60 |
49 |
| 11 |
TBPB (2.0) |
DMSO |
60 |
22 |
| 12 |
TBPB (2.0) |
DMF |
60 |
28 |
| 13 |
TBPB (2.0) |
Ethyl acetate |
60 |
Trace |
| 14 |
TBPB (2.0) |
Toluene |
60 |
21 |
| 15 |
TBPB (2.0) |
DCE |
60 |
27 |
| 16d |
TBPB (2.0) |
1,4-Dioxane |
60 |
ND |
| 17 |
TBPB (2.0) |
1,4-Dioxane |
50 |
42 |
| 18 |
TBPB (2.0) |
1,4-Dioxane |
80 |
33 |
| 19e |
TBPB (2.0) |
1,4-Dioxane |
60 |
53 |
| 20f |
TBPB (2.0) |
1,4-Dioxane |
60 |
55 |
| 21f |
TBPB (1.5) |
1,4-Dioxane |
60 |
61 |
Having these acceptable conditions in hand, we proceeded to examine the scope of the hydrophosphinylation using a variety of N-tethered 1,7-enynes 1 and diarylphosphine oxides. Upon performing the reaction using DPPO (2a), we were pleased to find that the substrates 1 with different substitution patterns on the aromatic rings of both the alkynyl (R2) and sulfonyl (R1) moieties could be efficiently converted into the corresponding poly-functionalized 3,4-dihydroquinolin-2(1H)-ones 3 in moderate to good yield. With a benzenesulfonyl protecting group (R1) on the amine anchor, a variety of substituents on the arylalkynyl moiety, including chloro, fluoro, methoxy and methyl, were tolerated under the above conditions. However, the electronic effect of the substituents on the arylalkynyl moiety had a significant impact on the reaction efficiency. For instance, substrates 1 possessing electron-withdrawing groups showed higher reactivity and gave higher obtainable yields than those with electron-donating counterparts (3b and 3cvs.3d and 3e). Furthermore, the electronic nature of the substituents on both the N-arylsulfonyl (R1) and arylalkynyl (R2) moieties was further investigated. The reaction occurred smoothly with a variety of functional groups on both the N-arylsulfonyl and arylalkynyl moieties of substrate 1. The reactions of substrates 1 involving N-arylsulfonyl moieties with electron-donating groups and arylalkynyl moieties attached with electron-withdrawing groups worked more efficiently to give access to the 3,4-dihydroquinolin-2(1H)-ones in 62% to 72% yield (3f, 3g, and 3j). Similarly, thienyl counterpart 1k was an adaptable substrate in this transformation, enabling the radical-initiated addition-cyclization and transforming into the corresponding 3,4-dihydroquinolin-2(1H)-one 3k in 51% yield. Moreover, substrates 1 bearing methyl, chloro, and fluoro groups on the N-phenyl moiety can also lead to the formation of 3,4-dihydroquinolin-2(1H)-ones 3l–3v, with yields ranging from 38%–64%.
Alternatively, bis(3,5-dimethylphenyl)phosphine oxide (2b) and di-p-tolylphosphine oxide (2c) were used to expand the scope of this radical addition-cyclization with 1,7-enynes 1g and 1w. As expected, both reactions proceeded readily to deliver the corresponding 3,4-dihydroquinolin-2(1H)-ones 3w and 3x in 66% and 62% yields, respectively. Moreover, a 1,7-enyne with a free amino group (1w) gave the expected 3,4-dihydroquinolin-2(1H)-ones 3y with 43% yield under the standard conditions. Unluckily, on replacing the aryl group with an n-butyl group on the alkynyl unit, 1,7-enyne 1x was not a good component for this reaction (Scheme 2, 3aa), which may be ascribed to the relative instability of the vinyl radical intermediate B (Scheme 4) generated in situ from P-centered radical triggered addition/6-exo-dig cyclization.
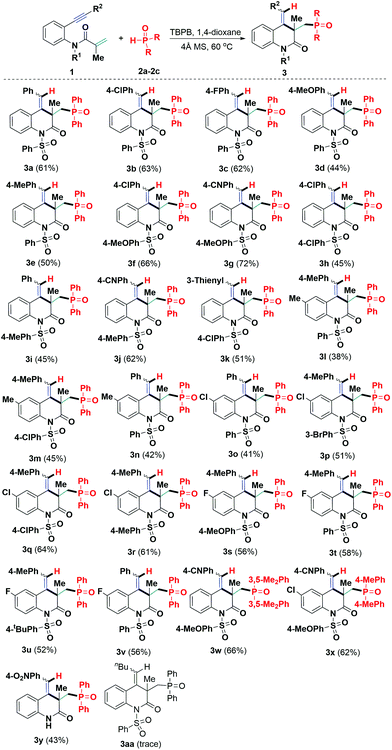 |
| | Scheme 2 Domino synthesis of the quinolin-2(1H)-ones 3. Yields for the isolated products are based on the 1,7-enynes 1 using column chromatography. Reaction conditions: 1 (0.3 mmol), 2 (1.2 mmol), TBPB (0.45 mmol), and dry 1,4-dioxane (2.0 mL), at 60 °C for 6.0 hours. | |
The structures provided for the resultant 3,4-dihydroquinolin-2(1H)-ones 3 were based on their NMR spectroscopy and HRMS results. In the case of 3l, its structure was readily confirmed using X-ray diffraction (Fig. 1 and see the ESI†).
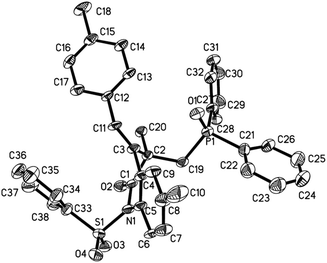 |
| | Fig. 1 X-ray structure of 3l. | |
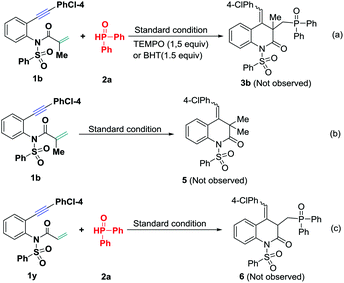
To gain mechanistic insight into this radical addition-cyclization, some control experiments were carried out. Treatment of 1,7-enyne 1b with DPPO 2a in the presence of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) or butylhydroxytoluene (BHT) (1.5 equiv.) under the standard conditions failed to give the desired product 3b, suggesting a possible radical mechanism (Scheme 3a). To confirm the phosphinylation and cyclization sequence, a reaction of 1b in the absence of DPPO 2a was conducted under the standard conditions, but without observation of 3,4-dihydroquinolin-2(1H)-one 5 (Scheme 3b), confirming a P-centered radical triggered α,β-conjugate addition/6-exo-dig cyclization to form a vinyl radical intermediate. These experimental results support that phosphinylation occurs prior to the cyclization step. Unfortunately, on exchanging the methyl group for hydrogen within the terminal olefin unit, no expected product 6 was observed with the starting material 1y remaining under the standard conditions (Scheme 3c), showing that the methyl group located in the terminal olefin unit plays a key role in the success of this reaction.
 |
| | Scheme 3 Control experiments. | |
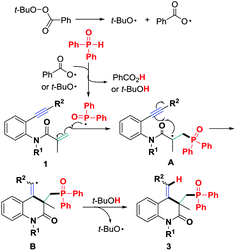
Based on the above results and the previously reported literature,12 a plausible mechanism is presented in Scheme 4. First, a P-centered radical is generated from diphenylphosphine oxide 2a through single-electron transfer (SET) mediated by the homolysis of TBPB. Subsequently, intermolecular α,β-conjugate addition of the resulting P-centered radical to the 1,7-conjugated enyne 1 and a subsequent 6-exo-dig cyclization generate the vinyl radical intermediate B,11b which is converted into the final 3,4-dihydroquinolin-2(1H)-one 3 by H-abstraction. Although P-centered radical triggered addition reactions have been realized successfully,12 hydrophosphinylation of 1,7-enynes toward polyfunctionalized 3,4-dihydroquinolin-2(1H)-ones via silver-free P-centered radical addition/6-exo-dig cyclization is unreported in organic chemistry as mentioned earlier.
 |
| | Scheme 4 Proposed mechanism for forming product 3. | |
Conclusions
In summary, we have developed a new silver-free P-centered radical-triggered 1,7-enyne-cyclization that provides an efficient synthesis for phosphorus-containing 3,4-dihydroquinolin-2(1H)-ones via cascade hydrophosphinylation. This reaction enables a sequential P-centered radical addition/6-exo-dig cyclization/H-abstraction process, allowing the successive formation of new C–P, C–C, and C–H bonds in a functional-group-compatible manner. The method offers a direct radical strategy for the synthesis of important phosphinylated compounds for potential applications in organic and medicinal chemistry. Currently, experiments toward further mechanistic studies are underway in our laboratory.
Experimental
General information
All the one-pot reactions were carried out in a 10 mL Schlenk tube equipped with a magnetic stir bar under air. All melting points are uncorrected. The NMR spectra were recorded in CDCl3 or DMSO-d6 on a 400 MHz instrument with TMS as the internal standard. Chemical shifts (δ) are reported in ppm with respect to TMS. Data are represented as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, m = multiplet), coupling constant (J, Hz) and integration. The HRMS analyses were carried out using a TOF-MS instrument with an ESI source. X-Ray crystallographic analysis was performed with a SMART CCD and a P4 diffractometer.
General procedure for the synthesis of product 3.
A mixture of the 1,7-enyne (1, 1.0 equiv., 0.3 mmol), diphenylphosphine oxide (2, 4.0 equiv., 1.2 mmol), 4 Å MS (200 mg) and TBPB (1.5 equiv.) in 1,4-dioxane (2.0 mL) was heated at 60 °C for 6 hours. After completion of the reaction, indicated using TLC, the mixture was dissolved in 20 ml of ethyl acetate and washed with saturated sodium bicarbonate solution (2 × 10 mL). The combined organic layers were washed with water (2 × 10 mL) and brine (1 × 10 mL). The solvent was removed under reduced pressure and the residue purified using column chromatography (petroleum ether/ethyl acetate) to afford the desired product as a white solid.
4-Benzylidene-3-((diphenylphosphoryl)methyl)-3-methyl-1-(phenylsulfonyl)-3,4-dihydroquinolin-2(1H)-one (3a).
White solid, mp 143–144 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 8.01–7.92 (m, 2H), 7.68–7.49 (m, 6H), 7.49–7.25 (m, 9H), 7.24–7.13 (m, 7H), 7.09 (s, 1H), 2.66–2.53 (m, 1H), 2.40–2.25 (m, 1H), 1.09 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 173.0 (d, J = 12.2 Hz), 139.5, 137.6, 134.7, 133.8, 133.7, 133.6 (d, J = 98.4 Hz), 133.5 (d, J = 99.6 Hz), 131.8 (d, J = 2.6 Hz), 131.4, 130.5, 130.4 (d, J = 1.7 Hz), 129.9, 128.7 (d, J = 12.7 Hz), 128.5 (d, J = 13.0 Hz), 128.4, 128.0 (d, J = 14.2 Hz), 127.7, 127.9, 127.7, 127.0, 126.9, 122.1, 50.72 (d, J = 3.6 Hz), 37.5 (d, J = 68.3 Hz), 24.3. IR (film, ν, cm−1) 3057, 2957, 1868, 1716, 1559, 1457, 1378, 1260, 1171, 1077, 962. HR-MS (ESI-TOF) m/z calcd for C36H30NO4PS [M + Na]+ 626.1531, found 626.1526.
4-(4-Chlorobenzylidene)-3-((diphenylphosphoryl)methyl)-3-methyl-1-(phenylsulfonyl)-3,4-dihydroquinolin-2(1H)-one (3b).
White solid, mp 117–118 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 7.96 (d, J = 8.0 Hz, 2H), 7.68–7.45 (m, 8H), 7.44–7.25 (m, 6H), 7.25–7.15 (m, 6H), 6.99 (s, 1H), 2.63–2.48 (m, 1H), 2.35–2.34 (m, 1H), 1.05 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 172.6 (d, J = 12.9 Hz), 139.4, 136.0, 135.4, 133.2 (d, J = 98.1 Hz), 132.5 (d, J = 104.0 Hz), 132.4, 131.7, 131.5, 131.4 (d, J = 5.5 Hz), 130.9 (d, J = 4.8 Hz), 130.4 (d, J = 9.0 Hz), 130.3 (d, J = 9.2 Hz), 129.1, 129.0, 128.8, 128.7, 128.6, 128.5 (d, J = 13.0 Hz), 128.2, 127.9, 127.6, 127.0, 122.1, 50.7 (d, J = 3.6 Hz), 37.0 (d, J = 68.5 Hz), 24.1. IR (film, ν, cm−1) 3059, 2947, 1905, 1716, 1599, 1489, 1367, 1171, 1016, 962, 809. HR-MS (ESI-TOF) m/z calcd for C36H29ClNO4PS [M + Na]+ 660.1141, found 660.1135.
3-((Diphenylphosphoryl)methyl)-4-(4-fluorobenzylidene)-3-methyl-1-(phenylsulfonyl)-3,4-dihydroquinolin-2(1H)-one (3c).
White solid, mp 142–143 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 8.01–7.96 (m, 2H), 7.70–7.53 (m, 6H), 7.52–7.36 (m, 5H), 7.36–7.29 (m, 3H), 7.27–7.17 (m, 5H), 7.03 (s, 1H), 6.99–6.92 (m, 2H), 2.64–2.54 (m, 1H), 2.35–2.25 (m, 1H), 1.08 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 172.7 (d, J = 13.3 Hz), 161.9 (d, J = 244.8 Hz), 139.4, 135.2, 133.8, 133.4 (d, J = 3.4 Hz), 132.3 (d, J = 99.1 Hz), 132.2 (d, J = 92.3 Hz), 131.8 (d, J = 2.0 Hz), 131.7, 131.5 (d, J = 1.5 Hz), 130.4 (d, J = 10.1 Hz), 130.3 (d, J = 9.1 Hz), 130.2 (d, J = 7.9 Hz), 128.8, 128.7, 128.6, 128.4, 128.0 (d, J = 17.3 Hz), 127.0, 122.1, 114.6 (d, J = 21.2 Hz), 50.7 (d, J = 3.6 Hz), 37.0 (d, J = 68.0 Hz), 23.9 (d, J = 3.8 Hz). IR (film, ν, cm−1) 3061, 3036, 1715, 1599, 1447, 1196, 1077, 1039, 961, 808. HR-MS (ESI-TOF) m/z calcd for C36H29FNO4PS [M + Na]+ 644.1437, found 644.1431.
3-((Diphenylphosphoryl)methyl)-4-(4-methoxybenzylidene)-3-methyl-1-(phenylsulfonyl)-3,4-dihydroquinolin-2(1H)-one (3d).
White solid, mp 108–109 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 8.02–7.95 (m, 2H), 7.69–7.58 (m, 3H), 7.57–7.52 (m, 3H), 7.52–7.29 (m, 8H), 7.27–7.16 (m, 5H), 7.06 (s, 1H), 6.81 (d, J = 8.4, 2H), 3.80 (s, 3H), 2.70–2.60 (m, 1H), 2.43–2.32 (m, 1H), 1.17 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 173.1 (d, J = 12.2 Hz), 158.6, 139.5, 134.5, 134.2, 133.3 (d, J = 92.5 Hz), 133.2 (d, J = 85.0 Hz), 133.1, 131.9, 131.8 (d, J = 2.5 Hz), 131.4 (d, J = 2.7 Hz), 130.5 (d, J = 9.5 Hz), 130.4 (d, J = 9.0 Hz), 130.0, 129.8, 128.9, 128.8, 128.6 (d, J = 11.8 Hz), 128.3, 127.9, 126.8, 121.9, 113.1, 55.2, 50.7 (d, J = 3.7 Hz), 37.4 (d, J = 68.2 Hz), 24.3 (d, J = 4.6 Hz). IR (film, ν, cm−1) 3058, 2950, 1966, 1712, 1508, 1398, 1283, 1171, 1083, 1028, 998, 810. HR-MS (ESI-TOF) m/z calcd for C37H32NO5PS [M + Na]+ 656.1637, found 656.1633.
3-((Diphenylphosphoryl)methyl)-3-methyl-4-(4-methylbenzylidene)-1-(phenylsulfonyl)-3,4-dihydroquinolin-2(1H)-one (3e).
White solid, mp 153–154 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 8.02–7.96 (m, 2H), 7.69–7.58 (m, 3H), 7.57–7.51 (m, 3H), 7.51–7.29 (m, 8H), 7.27–7.13 (m, 6H), 7.09 (s, 1H), 7.07 (s, 2H), 2.68–2.59 (m, 1H), 2.42–2.35 (m, 1H), 2.33 (s, 3H), 1.15 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 173.10 (d, J = 11.8 Hz), 139.5, 136.7, 134.6, 134.2, 133.7, 133.5 (d, J = 98.7 Hz), 133.4 (d, J = 98.7 Hz), 133.3, 131.9, 131.7 (d, J = 2.7 Hz), 131.3 (d, J = 2.6 Hz), 130.5 (d, J = 9.4 Hz), 130.4 (d, J = 9.0 Hz), 128.8, 128.6 (d, J = 12.1 Hz), 128.4, 128.3, 127.9, 127.8, 126.8, 122.0, 50.7 (d, J = 3.7 Hz), 37.6 (d, J = 68.5 Hz), 24.4 (d, J = 4.8 Hz), 21.20. IR (film, ν, cm−1) 3057, 2942, 1961, 1819, 1713, 1596, 1481, 1366, 1171, 1078, 962, 907, 807. HR-MS (ESI-TOF) m/z calcd for C37H32NO4PS [M + Na]+ 640.1688, found 640.1681.
4-(4-Chlorobenzylidene)-3-((diphenylphosphoryl)methyl)-1-((4-methoxyphenyl)sulfonyl)-3-methyl-3,4-dihydroquinolin-2(1H)-one (3f).
White solid, mp 123–124 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 7.90 (d, J = 9.2 Hz, 2H), 7.65–7.53 (m, 3H), 7.50–7.27 (m, 9H), 7.25–7.12 (m, 7H), 7.00–6.92 (m, 3H), 3.89 (s, 3H), 2.60–2.49 (m, 1H), 2.27 (dd, J = 15.0, 10.2, 1H), 1.07 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 172.6 (d, J = 13.2 Hz), 163.8, 136.0, 135.5, 132.9, 132.5 (d, J = 74.0 Hz), 131.7, 131.5 (d, J = 86.3 Hz), 130.5, 130.4 (d, J = 3.8 Hz), 130.3 (d, J = 3.9 Hz), 130.1, 128.7 (d, J = 12.4 Hz), 128.5 (d, J = 12.9 Hz), 128.1, 127.9 (d, J = 13.7 Hz), 126.9, 122.1, 113.9, 55.8, 50.7 (d, J = 3.3 Hz), 36.9 (d, J = 68.8 Hz), 24.0 (d, J = 3.1 Hz). IR (film, ν, cm−1) 3058, 2945, 1907, 1715, 1594, 1497, 1366, 1263, 1166, 1088, 1016, 960, 805. HR-MS (ESI-TOF) m/z calcd for C37H31ClNO5PS [M + Na]+ 690.1247, found 690.1241.
4-((3-((Diphenylphosphoryl)methyl)-1-((4-methoxyphenyl)sulfonyl)-3-methyl-2-oxo-2,3-dihydroquinolin-4(1H)-ylidene)methyl)benzonitrile (3g).
White solid, mp 198–200 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 7.91 (d, J = 8.8 Hz, 2H), 7.66–7.57 (m, 3H), 7.53 (s, 4H), 7.50–7.33 (m, 6H), 7.27 (s, 1H), 7.25–7.13 (m, 4H), 7.01–6.94 (m, 3H), 3.88 (s, 3H), 2.57–2.42 (m, 1H), 2.30–2.18 (m, 1H), 1.00 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 172.2 (d, J = 14.0 Hz), 163.9, 142.8, 136.4, 133.6 (d, J = 98.4 Hz), 133.5 (d, J = 99.2 Hz), 132.9, 132.0 (d, J = 2.6 Hz), 131.5 (d, J = 2.8 Hz), 131.4, 131.3, 131.0, 130.4 (d, J = 8.1 Hz), 130.2 (d, J = 9.6 Hz), 129.7, 128.7 (d, J = 11.8 Hz), 128.5, 128.4, 127.8, 127.0, 122.2, 118.9, 113.9, 110.8, 55.8, 50.8 (d, J = 3.3 Hz), 36.5 (d, J = 68.3 Hz), 23.6 (d, J = 2.0 Hz). IR (film, ν, cm−1) 3054, 2948, 2222, 1970, 1702, 1593, 1496, 1372, 1260, 1199, 1164, 1088, 962, 829. HR-MS (ESI-TOF) m/z calcd for C38H31N2O5PS [M + Na]+ 681.1589, found 681.1585.
4-(4-Chlorobenzylidene)-1-((4-chlorophenyl)sulfonyl)-3-((diphenylphosphoryl)methyl)-3-methyl-3,4-dihydroquinolin-2(1H)-one (3h).
White solid, mp 119–121 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 7.96–7.90 (m, 2H), 7.66–7.58 (m, 2H), 7.57–7.35 (m, 8H), 7.31–7.27 (m, 2H), 7.26–7.19 (m, 8H), 7.02 (s, 1H), 2.63–2.53 (m, 1H), 2.36–2.27 (m, 1H), 1.11 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 172.8 (d, J = 13.0 Hz), 140.6, 137.7, 135.9, 135.3, 134.0 (d, J = 2.4 Hz), 133.1, 132.1 (d, J = 103.6 Hz), 131.9 (d, J = 2.3 Hz), 131.8 (d, J = 91.1 Hz), 130.4 (d, J = 11.0 Hz), 130.3 (d, J = 9.2 Hz), 130.0, 129.1, 128.7, 128.6, 128.5 (d, J = 11.7 Hz), 128.2, 128.0, 127.1, 122.0, 50.7 (d, J = 3.2 Hz), 37.0 (d, J = 68.5 Hz), 24.2 (d, J = 3.7 Hz). IR (film, ν, cm−1) 3058, 2925, 1907, 1717, 1582, 1476, 1372, 1169, 1088, 962, 757. HR-MS (ESI-TOF) m/z calcd for C36H28Cl2NO4PS [M + Na]+ 694.0752, found 694.0754.
4-Benzylidene-3-((diphenylphosphoryl)methyl)-3-methyl-1-tosyl-3,4-dihydroquinolin-2(1H)-one (3i).
White solid, mp 133–134 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 7.87 (d, J = 8.4, 2H), 7.66–7.54 (m, 3H), 7.51–7.37 (m, 5H), 7.37–7.29 (m, 5H), 7.27–7.16 (m, 8H), 7.09 (s, 1H), 2.66–2.55 (m, 1H), 2.48 (s, 3H), 2.40–2.28 (m, 1H), 1.11 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 172.9 (d, J = 12.4 Hz), 144.9, 137.6, 136.5, 134.7, 133.8 (d, J = 98.4 Hz), 133.7, 133.6 (d, J = 98.7 Hz), 133.0, 131.9, 131.4 (d, J = 2.5 Hz), 131.3 (d, J = 2.5 Hz), 130.4 (d, J = 9.3 Hz), 129.4, 128.6, 128.5 (d, J = 9.5 Hz), 127.9 (d, J = 17.3 Hz), 127.7, 127.0, 126.8, 122.1, 50.7 (d, J = 3.5 Hz), 37.3 (d, J = 68.3 Hz), 24.1 (d, J = 4.2 Hz), 21.7. IR (film, ν, cm−1) 3055, 2921, 1964, 1714, 1599, 1455, 1438, 1365, 1170, 1078, 963, 811. HR-MS (ESI-TOF) m/z calcd for C37H32NO4PS [M + Na]+ 640.1688, found 640.1681.
4-((3-((Diphenylphosphoryl)methyl)-3-methyl-2-oxo-1-tosyl-2,3-dihydroquinolin-4(1H)-ylidene)methyl)benzonitrile (3j).
White solid, mp 143–144 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 7.85 (d, J = 8.4 Hz, 2H), 7.64–7.57 (m, 3H), 7.57–7.50 (m, 4H), 7.50–7.30 (m, 8H), 7.27 (s, 1H), 7.25–7.14 (m, 4H), 6.99 (s, 1H), 2.56–2.47 (m, 1H), 2.46 (s, 3H), 2.26–2.19 (m, 1H), 1.00 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 172.2 (d, J = 14.3 Hz), 145.1, 142.7, 136.3, 134.1, 132.9, 132.5 (d, J = 106.0 Hz), 132.4 (d, J = 103.0 Hz), 131.6, 131.4, 131.1 (d, J = 4.0 Hz), 130.9 (d, J = 4.3 Hz), 130.8 (d, J = 10.8 Hz), 130.3 (d, J = 9.4 Hz), 129.6, 129.4, 128.8, 128.7, 128.5 (d, J = 14.3 Hz), 127.8, 127.7, 127.0, 122.2, 118.8, 110.8, 50.8 (d, J = 3.6 Hz), 36.5 (d, J = 68.0 Hz), 23.6 (d, J = 2.7 Hz), 21.7. IR (film, ν, cm−1) 3059, 2948, 2225, 1715, 1600, 1500, 1401, 1366, 1196, 1088, 963, 811. HR-MS (ESI-TOF) m/z calcd for C38H31N2O4PS [M + Na]+ 665.1640, found 665.1636.
1-((4-Chlorophenyl)sulfonyl)-3-((diphenylphosphoryl)methyl)-3-methyl-4-(thiophen-3-ylmethylene)-3,4-dihydroquinolin-2(1H)-one (3k).
White solid, mp 131–132 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) = 7.99–7.91 (m, 2H), 7.65–7.57 (m, 2H), 7.54–7.46 (m, 4H), 7.46–7.37 (m, 4H), 7.36–7.29 (m, 4H), 7.27–7.21 (m, 3H), 7.15–7.09 (m, 1H), 7.05–7.01 (m, 2H), 6.90 (s, 1H), 2.70–2.63 (m, 1H), 2.49–2.39 (m, 1H), 1.28 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 173.3 (d, J = 11.9 Hz), 140.5, 137.9, 137.2, 135.7, 133.7 (d, J = 98.9 Hz), 133.2 (d, J = 98.7 Hz), 132.7, 131.8 (d, J = 2.6 Hz), 131.4 (d, J = 2.7 Hz), 130.5 (d, J = 9.5 Hz), 130.3 (d, J = 9.0 Hz), 129.9, 129.1, 128.9, 128.8, 128.7, 128.5 (d, J = 13.4 Hz), 128.1, 127.9, 126.8, 125.1, 123.7, 121.5, 50.6 (d, J = 3.6 Hz), 37.5 (d, J = 68.2 Hz), 24.0 (d, J = 5.2 Hz). IR (film, ν, cm−1) 3028, 2982, 1702, 1596, 1467, 1394, 1268, 1185, 1143, 1087, 1046, 1011, 961, 822. HR-MS (ESI-TOF) m/z calcd for C34H27ClNO4PS2 [M + Na]+ 666.0706, found 666.0701.
3-((Diphenylphosphoryl)methyl)-3,6-dimethyl-4-(4-methylbenzylidene)-1-(phenylsulfonyl)-3,4-dihydroquinolin-2(1H)-one (3l).
White solid, mp 152–153 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 7.99–7.93 (m, 2H), 7.67–7.57 (m, 3H), 7.56–7.45 (m, 4H), 7.45–7.29 (m, 7H), 7.19–7.12 (m, 3H), 7.10–7.03 (m, 3H), 6.96 (s, 1H), 2.67–2.59 (m, 1H), 2.40–2.34 (m, 1H), 2.33 (s, 6H), 1.15 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 172.9 (d, J = 12.6 Hz), 139.5, 136.7, 136.6, 134.6, 133.9, 133.8 (d, J = 98.5 Hz), 133.7 (d, J = 99.0 Hz), 133.6, 131.8, 131.7 (d, J = 2.5 Hz), 131.4 (d, J = 2.5 Hz), 130.5 (d, J = 9.0 Hz), 130.4 (d, J = 8.7 Hz), 130.0, 128.7, 128.6 (d, J = 11.6 Hz), 128.4, 128.3, 128.0, 122.0, 50.7 (d, J = 3.5 Hz), 37.3 (d, J = 68.2 Hz), 24.0 (d, J = 4.0 Hz), 21.2, 21.1. IR (film, ν, cm−1) 3056, 2919, 1715, 1509, 1447, 1367, 1195, 1130, 1099, 961, 829. HR-MS (ESI-TOF) m/z calcd for C38H34NO4PS [M + Na]+ 654.1844, found 654.1838.
1-((4-Chlorophenyl)sulfonyl)-3-((diphenylphosphoryl)methyl)-3,6-dimethyl-4-(4-methylbenzylidene)-3,4-dihydroquinolin-2(1H)-one (3m).
White solid, mp 184–186 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 7.94–7.87 (m, 2H), 7.63–7.56 (m, 2H), 7.53–7.46 (m, 3H), 7.45–7.39 (m, 4H), 7.39–7.29 (m, 4H), 7.19–7.08 (m, 5H), 7.03 (s, 1H), 6.95 (s, 1H), 2.67–2.58 (m, 1H), 2.41–2.36 (m, 1H), 2.34 (s, 3H), 2.33 (s, 3H), 1.19 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 173.0 (d, J = 11.9 Hz), 140.3, 137.9, 136.8, 136.7, 134.6, 134.5, 134.0, 133.8 (d, J = 98.6 Hz), 133.7 (d, J = 98.4 Hz), 131.8, 131.7 (d, J = 2.3 Hz), 131.4 (d, J = 2.1 Hz), 130.5 (d, J = 9.3 Hz), 130.4 (d, J = 8.9 Hz), 129.9, 129.0, 128.7 (d, J = 16.1 Hz), 128.5, 128.4, 128.3, 128.1, 122.0, 50.72 (d, J = 3.4 Hz), 37.4 (d, J = 68.1 Hz), 24.2 (d, J = 3.8 Hz), 21.2, 21.1. IR (film, ν, cm−1) 3056, 2920, 1914, 1720, 1580, 1475, 1375, 1201, 1172, 1091, 960, 755. HR-MS (ESI-TOF) m/z calcd for C38H33ClNO4PS [M + Na]+ 688.1454, found 688.1449.
4-Benzylidene-3-((diphenylphosphoryl)methyl)-3,6-dimethyl-1-(phenylsulfonyl)-3,4-dihydroquinolin-2(1H)-one (3n).
White solid, mp 166–167 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 7.99–7.93 (m, 2H), 7.69–7.58 (m, 3H), 7.57–7.51 (m, 2H), 7.50–7.45 (m, 2H), 7.44–7.29 (m, 7H), 7.27–7.15 (m, 6H), 7.07 (s, 1H), 6.98 (d, J = 1.2, 1H), 2.66–2.56 (m, 1H), 2.39–2.27 (m, 4H), 1.12 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 172.78 (d, J = 12.7 Hz), 139.5, 137.6, 136.8, 134.7, 133.7 (d, J = 98.3 Hz), 133.6 (d, J = 99.1 Hz), 131.7, 131.5 (d, J = 1.6 Hz), 131.4 (d, J = 3.7 Hz), 130.6, 130.5 (d, J = 9.3 Hz), 130.4 (d, J = 9.0 Hz), 129.9, 128.8, 128.7, 128.6, 128.5, 128.4, 128.3, 128.2 (d, J = 14.2 Hz) 127.7, 126.9, 122.1, 50.73 (d, J = 3.6 Hz), 37.2 (d, J = 68.6 Hz), 23.9, 21.1. IR (film, ν, cm−1) 3055, 2920, 1914, 1713, 1584, 1492, 1365, 1195, 1070, 961, 828. HR-MS (ESI-TOF) m/z calcd for C37H32NO4PS [M + Na]+ 640.1688, found 640.1681.
4-Benzylidene-6-chloro-3-((diphenylphosphoryl)methyl)-3-methyl-1-(phenylsulfonyl)-3,4-dihydroquinolin-2(1H)-one (3o).
White solid, mp 180–181 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 7.96 (d, J = 7.2, 2H), 7.70–7.58 (m, 3H), 7.58–7.51 (m, 2H), 7.51–7.38 (m, 6H), 7.38–7.27 (m, 4H), 7.26–7.14 (m, 5H), 7.08 (s, 1H), 7.00 (s, 1H) 2.70–2.55 (m, 1H), 2.41–2.26 (m, 1H), 1.13 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 172.9 (d, J = 12.0 Hz), 139.3, 137.1, 135.3, 133.9, 133.40 (d, J = 67.9 Hz), 132.8 (d, J = 60.0 Hz), 132.3, 131.9, 131.87 (d, J = 2.4 Hz), 131.7 (d, J = 2.5 Hz), 130.4 (d, J = 11.3 Hz), 130.3 (d, J = 10.9 Hz), 130.1, 128.9, 128.7 (d, J = 10.9 Hz), 128.5, 128.4, 128.3, 128.1, 127.9, 127.3, 123.1, 113.2, 50.53 (d, J = 3.7 Hz), 38.0 (d, J = 67.9 Hz), 24.5 (d, J = 4.2 Hz). IR (film, ν, cm−1) 3057, 2946, 1898, 1716, 1473, 1365, 1192, 1101, 1069, 959, 830. HR-MS (ESI-TOF) m/z calcd for C36H29ClNO4PS [M + Na]+ 660.1141, found 660.1135.
1-((3-Bromophenyl)sulfonyl)-6-chloro-3-((diphenylphosphoryl)methyl)-3-methyl-4-(4-methylbenzylidene)-3,4-dihydroquinolin-2(1H)-one (3p).
White solid, mp 117–118 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 8.09–8.03 (m, 1H), 7.95 (d, J = 8.0 Hz, 1H), 7.79–7.73 (m, 1H), 7.62–7.53 (m, 2H), 7.52–7.36 (m, 8H), 7.35–7.27 (m, 3H), 7.17 (d, J = 8.0 Hz, 2H), 7.10 (d, J = 8.0 Hz, 2H), 7.03 (s, 1H), 6.95 (s, 1H), 2.73–2.62 (m, 1H), 2.49–2.38 (m, 1H), 2.33 (s, 3H), 1.24 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 173.1 (d, J = 10.2 Hz), 141.1, 137.1, 136.9, 135.7, 133.6 (d, J = 84.1 Hz), 133.5 (d, J = 3.1 Hz), 131.9 (d, J = 85.4 Hz), 131.8, 130.7, 130.5, 130.4 (d, J = 3.1 Hz), 130.3, 128.6 (d, J = 11.7 Hz), 128.5 (d, J = 11.9 Hz), 128.2 (d, J = 17.6 Hz) 127.4, 127.1, 122.8, 122.7, 50.5 (d, J = 3.7 Hz), 38.3 (d, J = 67.6 Hz), 24.9 (d, J = 5.4 Hz), 21.2. IR (film, ν, cm−1) 3056, 2946, 1719, 1571, 1508, 1437, 1369, 1295, 1169, 1118, 1079, 961, 808. HR-MS (ESI-TOF) m/z calcd for C37H30BrClNO4PS [M + Na]+ 752.0403, found 752.0399.
6-Chloro-1-((4-chlorophenyl)sulfonyl)-3-((diphenylphosphoryl) methyl)-3-methyl-4-(4-methylbenzylidene)-3,4-dihydroquinolin-2(1H)-one (3q).
White solid, mp 202–203 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 7.98–7.92 (m, 2H), 7.63–7.56 (m, 2H), 7.55–7.41 (m, 9H), 7.40–7.30 (m, 4H), 7.13 (s, 3H), 7.01 (s, 1H), 6.93 (d, J = 2.4, 1H), 2.74–2.66 (m, 1H), 2.51–2.43 (m, 1H), 2.35 (s, 3H), 1.26 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 173.18 (d, J = 10.7 Hz), 140.6, 137.7, 137.2, 135.5, 134.0, 133.3 (d, J = 99.0 Hz), 133.2 (d, J = 98.4 Hz), 132.2, 131.9, 131.6, 130.5 (d, J = 6.7 Hz), 130.4 (d, J = 6.3 Hz), 130.0, 129.9 (d, J = 10.2 Hz), 129.2, 128.7, 128.6 (d, J = 10.5 Hz), 128.5 (d, J = 10.7 Hz), 128.3, 128.2, 128.1, 127.3, 122.9, 50.48 (d, J = 3.8 Hz), 38.3 (d, J = 68.2 Hz), 25.01 (d, J = 5.6 Hz), 21.2. IR (film, ν, cm−1) 3056, 2949, 1725, 1508, 1473, 1396, 1292, 1198, 1068, 959, 806. HR-MS (ESI-TOF) m/z calcd for C37H30Cl2NO4PS [M + Na]+ 708.0908, found 708.0903.
6-Chloro-3-((diphenylphosphoryl)methyl)-3-methyl-4-(4-methylbenzylidene)-1-tosyl-3,4-dihydroquinolin-2(1H)-one (3r).
White solid, mp 146–147 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 7.84 (d, J = 8.4 Hz, 2H), 7.61–7.53 (m, 2H), 7.50–7.35 (m, 7H), 7.35–7.25 (m, 5H), 7.13 (d, J = 8.0 Hz, 2H), 7.08 (d, J = 8.0 Hz, 2H), 6.97 (s, 1H), 6.88 (d, J = 2.4 Hz, 1H), 2.68–2.59 (m, 1H), 2.45 (s, 3H), 2.43–2.35 (m, 1H), 2.32 (s, 3H), 1.21 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 173.0 (d, J = 11.8 Hz), 145.0, 137.0, 136.26 (s, 2H), 135.4, 134.1, 133.8 (d, J = 99.8 Hz), 133.6, 133.4 (d, J = 98.8 Hz), 132.1, 131.8 (d, J = 2.7 Hz), 131.7 (d, J = 2.7 Hz), 131.6, 130.4 (d, J = 17.1 Hz), 130.3 (d, J = 16.7 Hz), 129.5, 128.7, 128.6, 128.5, 128.4 (d, J = 15.5 Hz), 128.0, 127.2, 123.1, 50.5 (d, J = 3.7 Hz), 38.0 (d, J = 68.1 Hz), 24.5 (d, J = 4.8 Hz), 21.7, 21.2. IR (film, ν, cm−1) 3058, 2945, 1910, 1718, 1597, 1478, 1395, 1295, 1194, 1076, 960, 875. HR-MS (ESI-TOF) m/z calcd for C38H33ClNO4PS [M + Na]+ 688.1454, found 688.1449.
3-((Diphenylphosphoryl)methyl)-6-fluoro-1-((4-methoxyphenyl)sulfonyl)-3-methyl-4-(4-methylbenzylidene)-3,4-dihydroquinolin-2(1H)-one (3s).
White solid, mp 165–166 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 7.93–7.87 (m, 2H), 7.65–7.57 (m, 2H), 7.54–7.30 (m, 9H), 7.18–7.07 (m, 4H), 7.06–6.96 (m, 4H), 6.68 (d, J = 6.4, 1H), 3.91 (s, 3H), 2.68–2.57 (m, 1H), 2.46–2.36 (m, 1H), 2.34 (s, 3H), 1.20 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 172.75 (d, J = 12.2 Hz), 163.9, 160.7 (d, J = 245.6 Hz), 136.9, 135.2, 133.9, 133.8, 133.6 (d, J = 98.7 Hz), 133.2 (d, J = 3.3 Hz), 133.0 (d, J = 98.0 Hz), 131.8 (d, J = 2.5 Hz), 131.7 (d, J = 2.7 Hz), 131.6, 131.5, 131.0, 130.9, 130.5 (d, J = 9.4 Hz), 130.2 (d, J = 9.3 Hz), 130.1, 129.0 (d, J = 2.8 Hz), 128.7, 128.6, 128.5, 128.3 (d, J = 3.3 Hz), 123.8 (d, J = 8.4 Hz), 114.8 (d, J = 22.8 Hz), 114.1 (d, J = 23.5 Hz), 113.9, 55.7, 50.5 (d, J = 3.8 Hz), 37.6 (d, J = 68.3 Hz), 24.3 (d, J = 4.3 Hz), 21.2. IR (film, ν, cm−1) 3056, 2923, 1713, 1593, 1494, 1365, 1229, 1166, 1024, 963, 832. HR-MS (ESI-TOF) m/z calcd for C38H33FNO5PS [M + Na]+ 688.1699, found 688.1696.
3-((Diphenylphosphoryl)methyl)-6-fluoro-3-methyl-4-(4-methylbenzylidene)-1-(phenylsulfonyl)-3,4-dihydroquinolin-2(1H)-one (3t).
White solid, mp 142–143 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 7.99–7.94 (m, 2H), 7.70–7.64 (m, 1H), 7.64–7.53 (m, 4H), 7.52–7.46 (m, 2H), 7.46–7.37 (m, 5H), 7.35–7.30 (m, 2H), 7.16 (d, J = 8.0, 2H), 7.10 (d, J = 8.0, 2H), 7.06–7.00 (m, 2H), 6.67–6.61 (m, 1H), 2.67–2.58 (m, 1H), 2.46–2.38 (m, 1H), 2.34 (s, 3H), 1.20 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 172.81 (d, J = 12.1 Hz), 160.8 (d, J = 245.7 Hz), 139.3, 137.0, 135.3, 134.0, 133.9, 133.8 (d, J = 99.0 Hz), 133.7, 133.2 (d, J = 99.0 Hz), 131.8 (d, J = 2.3 Hz), 131.7 (d, J = 2.2 Hz), 130.5 (d, J = 9.4 Hz), 130.3 (d, J = 9.0 Hz), 130.0, 129.2 (d, J = 6.1 Hz), 129.0, 128.8 (d, J = 13.9 Hz), 128.6, 128.5, 128.3 (d, J = 9.4 Hz), 123.7 (d, J = 8.4 Hz), 114.8 (d, J = 22.9 Hz), 114.2 (d, J = 23.4 Hz), 50.5 (d, J = 3.7 Hz), 37.7 (d, J = 68.1 Hz), 24.4 (d, J = 4.2 Hz), 21.2. IR (film, ν, cm−1) 3057, 2951, 1717, 1582, 1458, 1365, 1195, 1067, 962, 831. HR-MS (ESI-TOF) m/z calcd for C37H31FNO4PS [M + Na]+ 659.1593, found 658.1588.
1-((4-(tert-Butyl)phenyl)sulfonyl)-3-((diphenylphosphoryl)methyl)-6-fluoro-3-methyl-4-(4-methylbenzylidene)-3,4-dihydroquinolin-2(1H)-one (3u).
White solid, mp 109–111 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 8.18–8.10 (m, 1H), 7.91–7.85 (m, 2H), 7.66–7.59 (m, 2H), 7.58–7.54 (m, 2H), 7.52–7.46 (m, 3H), 7.45–7.38 (m, 4H), 7.34–7.31 (m, 1H), 7.12 (d, J = 8.0, 2H), 7.08–7.00 (m, 4H), 6.72 (d, J = 8.8, 1H), 2.71–2.60 (m, 1H), 2.46–2.34 (m, 1H), 2.32 (s, 3H), 1.38 (s, 9H), 1.18 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) δ 172.76 (d, J = 12.3 Hz), 160.8 (d, J = 245.6 Hz), 157.9, 136.9, 136.1, 135.2, 134.0 (d, J = 8.1 Hz), 133.8, 133.6 (d, J = 96.6 Hz), 133.3, 133.0 (d, J = 98.8 Hz), 131.8 (d, J = 2.6 Hz, 9H), 131.7 (d, J = 2.6 Hz), 130.5 (d, J = 9.4 Hz), 130.3 (d, J = 9.1 Hz), 130.1, 129.1 (d, J = 2.8 Hz), 128.6 (d, J = 11.9 Hz), 128.5, 128.4, 128.2, 125.8, 124.0 (d, J = 8.4 Hz), 114.8 (d, J = 22.8 Hz), 114.1 (d, J = 23.4 Hz), 50.5 (d, J = 3.8 Hz), 37.6 (d, J = 68.5 Hz), 35.3, 31.0, 24.3 (d, J = 4.5 Hz), 21.2. IR (film, ν, cm−1) 3056, 2961, 1716, 1592, 1458, 1366, 1268, 1193, 1082, 963, 856. HR-MS (ESI-TOF) m/z calcd for C41H39FNO4PS [M + Na]+ 714.2219, found 714.2212.
4-Benzylidene-3-((diphenylphosphoryl)methyl)-6-fluoro-3-methyl-1-(phenylsulfonyl)-3,4-dihydroquinolin-2(1H)-one (3v).
White solid, mp 155–157 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 8.00–7.92 (m, 2H), 7.71–7.65 (m, 1H), 7.65–7.52 (m, 5H), 7.51–7.37 (m, 6H), 7.37–7.29 (m, 4H), 7.27–7.19 (m, 3H), 7.08–7.00 (m, 2H), 6.72–6.65 (m, 1H), 2.69–2.55 (m, 1H), 2.45–2.32 (m, 1H), 1.17 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 172.6 (d, J = 12.5 Hz), 160.8 (d, J = 246.0 Hz), 139.3 (d, J = 8.9 Hz), 137.1, 135.1, 133.9, 133.8 (d, J = 98.4 Hz), 133.3 (d, J = 97.5 Hz), 131.8 (d, J = 2.1 Hz), 131.7 (d, J = 2.1 Hz), 130.5 (d, J = 9.3 Hz), 130.3 (d, J = 9.0 Hz), 129.0 (d, J = 2.9 Hz), 128.8, 128.7, 128.6, 128.4 (d, J = 11.0 Hz), 127.8, 127.2, 123.8 (d, J = 8.4 Hz), 114.9 (d, J = 22.8 Hz), 114.3 (d, J = 23.5 Hz), 50.5 (d, J = 3.7 Hz), 37.6 (d, J = 67.9 Hz), 24.2 (d, J = 4.0 Hz). IR (film, ν, cm−1) 3055, 2944, 1716, 1589, 1484, 1365, 1262, 1168, 1085, 1027, 963, 831. HR-MS (ESI-TOF) m/z calcd for C36H29FNO4PS [M + Na]+ 644.1437, found 644.1431.
4-((3-((Bis(3,5-dimethylphenyl)phosphoryl)methyl)-1-((4-methoxyphenyl)sulfonyl)-3-methyl-2-oxo-2,3-dihydroquinolin-4(1H)-ylidene)methyl)benzonitrile (3w).
White solid, mp 146–147 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 7.93 (d, J = 8.4 Hz, 2H), 7.66 (d, J = 8.0 Hz, 1H), 7.61–7.52 (m, 4H), 7.45–7.34 (m, 2H), 7.29 (s, 2H), 7.25–7.15 (m, 4H), 7.13–6.95 (m, 6H), 6.87 (d, J = 12.0 Hz, 2H), 3.91 (s, 3H), 2.53–2.43 (m, 1H), 2.42–2.34 (m, 1H), 2.32 (s, 6H), 2.24 (s, 6H), 1.01 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) δ 172.3 (d, J = 14.5 Hz), 163.9, 142.8, 138.4 (d, J = 12.3 Hz), 138.2 (d, J = 12.3 Hz), 136.4 (d, J = 2.3 Hz), 136.3, 133.6 (d, J = 97.6 Hz), 133.5 (d, J = 2.7 Hz), 133.4 (d, J = 98.5 Hz), 133.3 (d, J = 2.7 Hz), 132.9, 131.3, 131.2 (d, J = 38.0 Hz), 130.5, 129.7, 127.9 (d, J = 38.9 Hz), 127.8 (d, J = 11.4 Hz), 126.8, 122.2, 118.9, 113.9, 110.7, 55.8, 50.8 (d, J = 3.6 Hz), 36.4 (d, J = 67.3 Hz), 23.5, 21.3(4), 21.3(27). IR (film, ν, cm−1) 3063, 2946, 2227, 1716, 1595, 1498, 1366, 1263, 1166, 1087, 962, 833. HR-MS (ESI-TOF) m/z calcd for C42H39N2O5PS [M + Na]+ 737.2215, found 737.2211.
6-Chloro-1-((4-chlorophenyl)sulfonyl)-3-((di-p-tolylphosphoryl)methyl)-3-methyl-4-(4-methylbenzylidene)-3,4-dihydroquinolin-2(1H)-one (3x).
White solid, mp 128–129 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 7.97–7.89 (m, 2H), 7.53–7.48 (m, 2H), 7.45–7.38 (m, 3H), 7.30–7.27 (m, 2H), 7.26–7.19 (m, 3H), 7.17–7.08 (m, 6H), 6.96 (s, 1H), 6.77 (d, J = 2.4 Hz, 1H), 2.65–2.56 (m, 1H), 2.46–2.38 (m, 1H), 2.36 (s, 3H), 2.35 (s, 3H), 2.33 (s, 3H), 1.26 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 173.23 (d, J = 11.6 Hz), 142.3 (d, J = 2.7 Hz), 142.2 (d, J = 2.8 Hz), 140.6, 137.8, 137.1, 135.6, 134.0, 133.5 (d, J = 2.7 Hz), 133.2, 132.2, 131.5, 130.5 (d, J = 9.8 Hz), 130.4 (d, J = 101.3 Hz), 130.3 (d, J = 9.4 Hz), 129.8, 129.7, 129.6, 129.5, 129.4 (d, J = 12.0 Hz), 129.3 (d, J = 12.1 Hz), 129.2, 128.6, 127.8 (d, J = 104.8 Hz), 122.8, 50.5 (d, J = 3.7 Hz), 38.3 (d, J = 67.9 Hz), 24.7 (d, J = 5.2 Hz), 21.6, 21.5, 21.2. IR (film, ν, cm−1) 3051, 1718, 1601, 1475, 1370, 1294, 1187, 1089, 1013, 960, 852. HR-MS (ESI-TOF) m/z calcd for C39H34Cl2NO4PS [M + Na]+ 736.1221, found 736.1217.
3-((Diphenylphosphoryl)methyl)-3-methyl-4-(4-nitrobenzylidene)-3,4-dihydroquinolin-2(1H)-one (3y).
White solid, mp 179–180 °C; 1H NMR (400 MHz, CDCl3; δ, ppm) 8.19–8.13 (m, 2H), 8.02–7.98 (m, 1H), 7.97 (d, J = 8.4 Hz, 1H), 7.66–7.55 (m, 4H), 7.54–7.41 (m, 6H), 7.41–7.29 (m, 5H), 3.33–3.18 (m, 2H), 1.57 (s, 3H). 13C NMR (100 MHz, CDCl3; δ, ppm) 194.1 (d, J = 1.6 Hz), 173.1, 139.6, 138.7, 134.9, 134.1 (d, J = 99.9 Hz), 133.9 (d, J = 100.9 Hz), 133.8, 131.6 (d, J = 2.9 Hz), 131.3 (d, J = 2.8 Hz), 130.8 (d, J = 9.9 Hz), 130.7 (d, J = 9.8 Hz), 128.8, 128.6, 128.4 (d, J = 12.4 Hz), 128.3, 128.1, 125.4, 121.4, 120.3, 57.6 (d, J = 4.4 Hz), 35.4 (d, J = 68.3 Hz), 26.4 (d, J = 13.1 Hz). IR (film, ν, cm−1) 3057, 2966, 2871, 2218, 1963, 1720, 1685, 1596, 1478, 1459, 1365, 1320, 1178, 1082, 905, 802. HR-MS (ESI) m/z calcd for C30H25N2O4P [M + Na]+ 531.1450, found 531.1439.
Acknowledgements
We are grateful for financial support from the NSFC (no. 21232004, 21272095, and 21472071), the PAPD of Jiangsu Higher Education Institutions, the Outstanding Youth Fund of JSNU (YQ2015003), the NSF of Jiangsu Province (BK20151163), and the Open Foundation of Jiangsu Key Laboratory (K201505).
References
-
(a)
D. E. C. Corbridge, Phosphorus: Chemistry, Biochemistry and Technology, CRC Press, London, 2013 Search PubMed;
(b)
D. T. Kolio, Chemistry and Application of H-Phosphonates, Elsevier Science, Oxford, 2006 Search PubMed;
(c) C. Giorgio, O. Gianmauro and A. P. Gerard, Tetrahedron, 2003, 59, 9471 CrossRef;
(d) W. Tang and X. Zhang, Chem. Rev., 2003, 103, 3029 CrossRef CAS PubMed;
(e)
L. D. Quin, A Guide to Organophosphorus Chemistry, Wiley-Interscience, Hoboken, NJ, 2000 Search PubMed;
(f) A. K. Bhattacharta and G. Thyagarajan, Chem. Rev., 1981, 81, 415 CrossRef;
(g)
G. M. Kosolapoff and L. Maier, Organic Phosphorus Compounds, Wiley-Interscience, New York, 1972 Search PubMed.
-
(a) X. Chen, D. J. Kopecky, J. Mihalic, S. Jeffries, X. Min, J. Heath, J. Deignan, S. Lai, Z. Fu, C. Guimaraes, S. Shen, S. Li, S. Johnstone, S. Thibault, H. Xu, M. Cardozo, W. Shen, N. Walker, F. Kayser and Z. Wang, J. Med. Chem., 2012, 55, 3837 CrossRef CAS PubMed;
(b) Q. Dang, Y. Liu, D. K. Cashion, S. R. Kasib-hatla, T. Jiang, F. Taplin, J. D. Jacintho, H. Li, Z. Sun, Y. Fan, J. DaRe, F. Tian, W. Li, T. Gibson, R. Lemus, P. D. van Poelje, S. C. Potter and M. D. Erion, J. Med. Chem., 2011, 54, 153 CrossRef CAS PubMed;
(c) S. Sivendran, V. Jones, D. Sun, Y. Wang, A. E. Grzegorzewicz, M. S. Scherman, A. D. Napper, J. A. McCammon, R. E. Lee, S. L. Diamond and M. McNeil, Bioorg. Med. Chem., 2010, 18, 896 CrossRef CAS PubMed.
-
(a) V. Spampinato, N. Tuccitto, S. Quici, V. Calabrese, G. Marletta, A. Torrisi and A. Licciardello, Langmuir, 2010, 26, 8400 CrossRef CAS PubMed;
(b) S. Kirumakki, J. Huang, A. Subbiah, J. Yao, A. Rowland, B. Smith, A. Mukherjee, S. Samarajeewa and A. Clearfield, J. Mater. Chem., 2009, 19, 2593 RSC;
(c) H. Steininger, M. Schuster, K. D. Kreuer, A. Kaltbeitzel, B. Bingol, W. H. Meyer, S. Schauff, G. Brunklaus, J. Maier and H. W. Spiess, Phys. Chem. Chem. Phys., 2007, 9, 1764 RSC.
- Selected reviews see:
(a) W. Tang and X. Zhang, Chem. Rev., 2003, 103, 3029 CrossRef CAS PubMed;
(b)
Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, Weinheim, 2004 Search PubMed;
(c)
Phosphorus Ligands in Asymmetric Catalysis-Synthesis and Application, ed. A. Borner, Wiley-VCH, Weinheim, 2008 Search PubMed;
(d) M. N. Birkholz, Z. Freixa and P. W. N. M. van Leeuwen, Chem. Soc. Rev., 2009, 38, 1099 RSC;
(e) H. Fernandez-Perez, P. Etayo, A. Panossian and A. Vidal-Ferran, Chem. Rev., 2011, 111, 2119 CrossRef CAS PubMed;
(f)
Phosphorus (III) Ligands in Homogeneous Catalysis: Design and Synthesis, ed. P. C. J. Kamer and P. W. N. M. van Leeuwen, Wiley-VCH, Chichester, UK, 2012 Search PubMed.
-
(a) T. Oshiki and T. Imamoto, J. Am. Chem. Soc., 1992, 114, 3975 CrossRef CAS;
(b) J.-L. Montchamp and Y. R. Dumond, J. Am. Chem. Soc., 2001, 123, 510 CrossRef CAS;
(c) J. R. Moncarz, N. F. Laritcheva and D. S. Glueck, J. Am. Chem. Soc., 2002, 124, 13356 CrossRef CAS PubMed;
(d) J. R. Moncarz, T. J. Brunker, D. S. Glueck, R. D. Sommer and A. L. Rheingold, J. Am. Chem. Soc., 2003, 125, 1180 CrossRef CAS PubMed;
(e) D. Gelman, L. Jiang and S. L. Buchwald, Org. Lett., 2003, 5, 2315 CrossRef CAS PubMed;
(f) V. S. Chan, R. G. Bergman and F. D. Toste, J. Am. Chem. Soc., 2007, 129, 15122 CrossRef CAS PubMed;
(g) Y. Gao, G. Wang, L. Chen, P. Xu, Y. Zhao, Y. Zhou and L.-B. Han, J. Am. Chem. Soc., 2009, 131, 7956 CrossRef CAS PubMed;
(h) R. Zhuang, J. Xu, Z. Cai, G. Tang, M. Fang and Y. Zhao, Org. Lett., 2011, 13, 2110 CrossRef CAS PubMed;
(i) C. Hou, Y. Ren, R. Lang, X. Hu, C. Xia and F. Li, Chem. Commun., 2012, 5181 RSC;
(j) C.-G. Feng, M. Ye, K.-J. Xiao, S. Li and J.-Q. Yu, J. Am. Chem. Soc., 2013, 135, 9322 CrossRef CAS PubMed;
(k) J. Hu, N. Zhao, B. Yang, G. Wang, L.-N. Guo, Y.-M. Liang and S.-D. Yang, Chem. – Eur. J., 2011, 17, 5516 CrossRef CAS PubMed.
- For selected examples, see:
(a) L.-B. Han and M. Tanaka, J. Am. Chem. Soc., 1996, 118, 1571 CrossRef CAS;
(b) O. Tayama, A. Nakano, T. Iwahama, S. Sakaguchi and Y. Ishii, J. Org. Chem., 2004, 69, 5494 CrossRef CAS PubMed;
(c) L.-B. Han, C. Zhang, H. Yazawa and S. Shimada, J. Am. Chem. Soc., 2004, 126, 5080 CrossRef CAS PubMed;
(d) T. Hirai and L.-B. Han, Org. Lett., 2007, 9, 53 CrossRef CAS PubMed;
(e) V. P. Ananikov, L. L. Khemchyan and I. P. Beletskaya, Synlett, 2009, 2375 CrossRef CAS;
(f) J.-J. Feng, X.-F. Chen, M. Shi and W.-L. Duan, J. Am. Chem. Soc., 2010, 132, 5562 CrossRef CAS PubMed.
- For selected examples, see:
(a) F. Beaufils, F. Denes and P. Renaud, Angew. Chem., Int. Ed., 2005, 44, 5273 CrossRef CAS PubMed;
(b) L.-B. Han and C.-Q. Zhao, J. Org. Chem., 2005, 70, 10121 CrossRef CAS PubMed;
(c) X.-Q. Pan, J.-P. Zou, G.-L. Zhang and W. Zhang, Chem. Commun., 2010, 46, 1721 RSC;
(d) X.-Q. Pan, L. Wang, J.-P. Zou and W. Zhang, Chem. Commun., 2011, 47, 7875 RSC;
(e) C. Zhang, Z. Li, L. Zhu, L. Yu, Z. Wang and C. Li, J. Am. Chem. Soc., 2013, 135, 14082 CrossRef CAS PubMed;
(f) X.-Q. Chu, Y. Zi, H. Meng, X.-P. Xu and S.-J. Ji, Chem. Commun., 2014, 50, 7642 RSC;
(g) X. Mi, C. Wang, M. Huang, J. Zhang, Y. Wu and Y. Wu, Org. Lett., 2014, 16, 3356 CrossRef CAS PubMed;
(h) Y.-M. Li, M. Sun, H.-L. Wang, Q.-P. Tian and S.-D. Yang, Angew. Chem., Int. Ed., 2013, 52, 3972 CrossRef CAS PubMed.
- For selected reviews:
(a) B. M. Trost, Acc. Chem. Res., 1990, 23, 34 CrossRef CAS;
(b) C. Aubert, O. Buisine and M. Malacria, Chem. Rev., 2002, 102, 813 CrossRef CAS PubMed;
(c) C. Bruneau, Angew. Chem., Int. Ed., 2005, 44, 2328 CrossRef CAS PubMed;
(d) L. Zhang, J. Sun and S. Kozmin, Adv. Synth. Catal., 2006, 348, 2271 CrossRef CAS;
(e) J. Marco-Contelles and E. Soriano, Chem. – Eur. J., 2007, 13, 1350 CrossRef CAS PubMed;
(f) A. Fuerstner and P. W. Davies, Angew. Chem., Int. Ed., 2007, 46, 3410 CrossRef CAS PubMed;
(g) S. I. Lee and N. Chatani, Chem. Commun., 2009, 371 RSC;
(h) I. D. G. Watson and F. D. Toste, Chem. Sci., 2012, 3, 2899 RSC;
(i) U. Wille, Chem. Rev., 2013, 113, 813 CrossRef CAS PubMed.
- For selected examples of 1,n-enyne cyclization, see:
(a) P. Liu, Y. Fukui, P. Tian, Z.-T. He, C.-Y. Sun, N.-Y. Wu and G.-Q. Lin, J. Am. Chem. Soc., 2013, 135, 11700 CrossRef CAS PubMed;
(b) C. Fehr and J. Galindo, Angew. Chem., Int. Ed., 2006, 45, 2901 CrossRef CAS PubMed;
(c) A. Fuerstner, R. Martin and K. Majima, J. Am. Chem. Soc., 2005, 127, 12236 CrossRef CAS PubMed;
(d) J. M. O'Conner, S. J. Friese and B. L. Rodgers, J. Am. Chem. Soc., 2005, 127, 16342 CrossRef PubMed;
(e) K. T. Sylvester and P. J. Chirik, J. Am. Chem. Soc., 2009, 131, 8772 CrossRef CAS PubMed;
(f) B. M. Trost and J. M. Tour, J. Am. Chem. Soc., 1987, 109, 5268 CrossRef CAS;
(g) M. Chen, Y. Weng, M. Guo, H. Zhang and A. Lei, Angew. Chem., Int. Ed., 2008, 47, 2279 CrossRef CAS PubMed.
- For selected examples, see:
(a) M. Hu, R.-J. Song and J.-H. Li, Angew. Chem., Int. Ed., 2015, 54, 608 CAS;
(b) Y. Liu, J.-L. Zhang, R.-J. Song, P.-C. Qian and J.-H. Li, Angew. Chem., Int. Ed., 2014, 53, 9017 CrossRef CAS PubMed;
(c) G.-B. Deng, Z.-Q. Wang, J.-D. Xia, P.-C. Qian, R.-J. Song, M. Hu, L.-B. Gong and J.-H. Li, Angew. Chem., Int. Ed., 2013, 52, 1535 CrossRef CAS PubMed;
(d) N. Fuentes, W. Kong, L. Fernández-Sánchez, E. Merino and C. Nevado, J. Am. Chem. Soc., 2015, 137, 964 CrossRef CAS PubMed;
(e) Y.-Q. Wang, Y.-T. He, L.-L. Zhang, X.-X. Wu, X.-Y. Liu and Y.-M. Liang, Org. Lett., 2015, 17, 4280 CrossRef CAS PubMed;
(f) Y.-F. Qiu, X.-Y. Zhu, Y.-X. Li, Y.-T. He, F. Yang, J. Wang, H.-L. Hua, L. Zheng, L.-C. Wang, X.-Y. Liu and Y.-M. Liang, Org. Lett., 2015, 17, 3694 CrossRef CAS PubMed.
-
(a) Z.-Z. Chen, S. Liu, W.-J. Hao, G. Xu, S. Wu, J.-N. Miao, B. Jiang, S.-L. Wang, S.-J. Tu and G. Li, Chem. Sci., 2015, 6, 6654 RSC;
(b) J.-K. Qiu, B. Jiang, Y.-L. Zhu, W.-J. Hao, D.-C. Wang, J. Sun, P. Wei, S.-J. Tu and G. Li, J. Am. Chem. Soc., 2015, 137, 8928 CrossRef CAS PubMed.
- For selected examples, see:
(a) M. Hu, J.-H. Fan, Y. Liu, X.-H. Ouyang, R.-J. Song and J.-H. Li, Angew. Chem., Int. Ed., 2015, 54, 9577 CrossRef CAS PubMed;
(b) A. Kamimura, T. Yoshinaga, F. Noguchi, K. Miyazaki and H. Uno, Org. Chem. Front., 2015, 2, 713 RSC;
(c) X.-H. Hao, P. Gao, X.-R. Song, Y.-F. Qiu, D.-P. Jin, X.-Y. Liu and Y.-M. Liang, Chem. Commun., 2015, 51, 6839 RSC;
(d) J.-Y. Luo, H.-L. Hua, Z.-S. Chen, Z.-Z. Zhou, Y.-F. Yang, P.-X. Zhou, Y.-T. He, X.-Y. Liu and Y.-M. Liang, Chem. Commun., 2014, 50, 1564 RSC;
(e) X. Zeng, L. Ilies and E. Nakamura, Org. Lett., 2012, 14, 954 CrossRef CAS PubMed;
(f) T. Taniguchi, N. Goto, A. Nishibata and H. Ishibashi, Org. Lett., 2010, 12, 5084 CrossRef CAS;
(g) T. Taniguchi, N. Goto, A. Nishibata and H. Ishibashi, Org. Lett., 2010, 12, 112 CrossRef CAS PubMed;
(h) Z.-Z. Zhou, D.-P. Jin, L.-H. Li, Y.-T. He, P.-X. Zhou, X.-B. Yan, X.-Y. Liu and Y.-M. Liang, Org. Lett., 2014, 16, 5616 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1434265 (3l). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qo00430f |
| ‡ These authors contributed equally to this work. |
|
| This journal is © the Partner Organisations 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond systems in a single step fashion. Specifically, oxidative radical 1,n-enyne-cyclizations have gradually become a powerful tool for rapidly constructing cyclic compounds.10 These reactions not only feature high atom-economy, annulation efficiency, and excellent functional group compatibility but also avoid prefunctionalization of the reaction substrates or the use of expensive transition metals. Recently, our groups11 and others12 have developed a series of unique radical 1,n-enyne-cyclizations for the construction of functionalized complex molecules of chemical and pharmaceutical interest. For instance, Liang and coworkers reported a phosphorus radical-involved 1,6-enyne-cyclization reaction to synthesize fluorene derivatives (Scheme 1c).12h However, in sharp contrast, phosphorus radical triggered 1,7-enyne-cyclizations are virtually unexplored partly because of the poor selectivity of the radical addition toward alkenyl (Scheme 1e, path a) and alkynyl (Scheme 1e, path b) groups. Thus, regioselective radical addition-cyclization of 1,7-enynes is highly challenging. Very recently, we developed the addition of various C-centered radicals to N-tethered 1,7-enynes 1, which underwent a radical addition-cyclization/H-abstraction/radical coupling sequence to give access to spirosubstituted cyclopenta[c]quinolones (Scheme 1d).11a We envisioned that, under the right set of oxidative conditions, phosphorus radicals could be preferentially engaged in alkenyl addition with the N-tethered 1,7-enynes 1 to control the reaction selectivity (Scheme 1e, path a), and thereby afford functionalized 3,4-dihydroquinolin-2(1H)-ones. Herein we report the successful implementation of this concept with a new cascade phosphorus-radical triggered 1,7-enyne-cyclization, which enabled the formation of a variety of phosphorus-containing 3,4-dihydroquinolin-2(1H)-ones through hydrophosphinylation of 1,7-enynes in a highly controlled manner (Scheme 1e).
C bond systems in a single step fashion. Specifically, oxidative radical 1,n-enyne-cyclizations have gradually become a powerful tool for rapidly constructing cyclic compounds.10 These reactions not only feature high atom-economy, annulation efficiency, and excellent functional group compatibility but also avoid prefunctionalization of the reaction substrates or the use of expensive transition metals. Recently, our groups11 and others12 have developed a series of unique radical 1,n-enyne-cyclizations for the construction of functionalized complex molecules of chemical and pharmaceutical interest. For instance, Liang and coworkers reported a phosphorus radical-involved 1,6-enyne-cyclization reaction to synthesize fluorene derivatives (Scheme 1c).12h However, in sharp contrast, phosphorus radical triggered 1,7-enyne-cyclizations are virtually unexplored partly because of the poor selectivity of the radical addition toward alkenyl (Scheme 1e, path a) and alkynyl (Scheme 1e, path b) groups. Thus, regioselective radical addition-cyclization of 1,7-enynes is highly challenging. Very recently, we developed the addition of various C-centered radicals to N-tethered 1,7-enynes 1, which underwent a radical addition-cyclization/H-abstraction/radical coupling sequence to give access to spirosubstituted cyclopenta[c]quinolones (Scheme 1d).11a We envisioned that, under the right set of oxidative conditions, phosphorus radicals could be preferentially engaged in alkenyl addition with the N-tethered 1,7-enynes 1 to control the reaction selectivity (Scheme 1e, path a), and thereby afford functionalized 3,4-dihydroquinolin-2(1H)-ones. Herein we report the successful implementation of this concept with a new cascade phosphorus-radical triggered 1,7-enyne-cyclization, which enabled the formation of a variety of phosphorus-containing 3,4-dihydroquinolin-2(1H)-ones through hydrophosphinylation of 1,7-enynes in a highly controlled manner (Scheme 1e).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (1a
4 (1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a) using 1,4-dioxane at 60 °C further facilitated the reaction process and improved the yield of 3a to 61% (entry 21).
2a) using 1,4-dioxane at 60 °C further facilitated the reaction process and improved the yield of 3a to 61% (entry 21).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a was 1
2a was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.
f The ratio of 1a
3.
f The ratio of 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a was 1
2a was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.
4.






