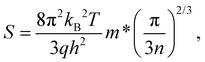Enhanced thermoelectric properties of the Dirac semimetal Cd3As2†
Tong
Zhou
ab,
Cheng
Zhang
ac,
Huisheng
Zhang
ab,
Faxian
Xiu
ac and
Zhongqin
Yang
*abc
aState Key Laboratory of Surface Physics and Department of Physics, Fudan University, Shanghai 200433, China. E-mail: zyang@fudan.edu.cn
bKey Laboratory for Computational Physical Sciences (MOE), Fudan University, Shanghai 200433, China
cCollaborative Innovation Center of Advanced Microstructures, Fudan University, Shanghai, 200433, China
First published on 1st November 2016
Abstract
We report an investigation of temperature- and doping-dependent thermoelectric behavior of the topological semimetal Cd3As2. The electrical conductivity, thermal conductivity, Seebeck coefficient, and figure of merit (ZT) are calculated using the Boltzmann transport theory. The calculated thermoelectric properties of the pristine Cd3As2 match well with the experimental results. Electron or hole doping, especially the latter, is found to much improve the thermoelectric behavior of the material. The optimum figure of merit ZT of Cd3As2 with electron doping is found to be about 0.5 at T = 700 K with n = 1 × 1020 cm−3, which is much larger than the maximum experimental value obtained for pristine Cd3As2 (∼0.15). For p-type Cd3As2, the maximal value of the Seebeck coefficient as a function of temperature increases apparently with the increase of the hole doping concentration and its position shifts drastically towards the lower temperature region, compared to that of n-type Cd3As2. This leads to an optimum figure of merit ZT of about 0.5, obtained at a low temperature of 500 K (p = 1 × 1020 cm−3) in the p-type Cd3As2.
I. Introduction
Materials exhibiting thermoelectric performance are of considerable importance in industry due to their numerous potential applications, including in vehicular exhaust waste-heat recovery, energy harvesting, heating and cooling, and solid-state energy conversion.1,2 High thermoelectric performance is extremely desirable for these applications.3,4 Thermoelectric behavior is typically quantified in terms of a dimensionless figure of merit (ZT) given by the expression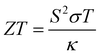 , in which S is the Seebeck coefficient or thermopower, σ is the electrical conductivity, T is the absolute temperature, and κ is the thermal conductivity, normally the sum of the electronic and lattice contributions, namely κ = κe + κL. S2σ is the power factor of the thermoelectric performance. The ZT expression shows that for good thermoelectric performance both the Seebeck coefficient and electrical conductivity are expected to be high, while the thermal conductivity is expected to be low. These parameters are, however, intimately coupled to each other, resulting in conflicts in the optimization process.1,2 Hence, optimizing and finding usable high-performance thermoelectric materials remains full of challenges.
, in which S is the Seebeck coefficient or thermopower, σ is the electrical conductivity, T is the absolute temperature, and κ is the thermal conductivity, normally the sum of the electronic and lattice contributions, namely κ = κe + κL. S2σ is the power factor of the thermoelectric performance. The ZT expression shows that for good thermoelectric performance both the Seebeck coefficient and electrical conductivity are expected to be high, while the thermal conductivity is expected to be low. These parameters are, however, intimately coupled to each other, resulting in conflicts in the optimization process.1,2 Hence, optimizing and finding usable high-performance thermoelectric materials remains full of challenges.
Recently, three-dimensional (3D) Dirac semimetal states have been theoretically predicted and experimentally realized in Cd3As2.5–9 Unlike other semimetals, the Cd3As2 crystal possesses Dirac fermions that disperse linearly in k-space and as a result it becomes a crystalline material with an ultrahigh electron mobility, μ, of about 104–106 cm2 V−1 s−1.8,9 Since the power factor strongly depends on the electron mobility i.e., S2σ ≈ μ(m*/me)1.5 (where m* is the energy-band electron effective mass and me is the free electron mass),10 Cd3As2 shows great potential for high performance thermoelectric applications. The maximum ZT value of pristine Cd3As2 that has been achieved in experiments is, however, only about 0.15.11,12 How to improve the thermoelectric performance in this material is of significance. Semimetals usually can be easily doped with both electrons and holes due to the existence of Dirac cones near the Fermi level (EF), which has been proved in graphene.13 Both the n-type11,12,14,15 and p-type16,17 Cd3As2 have also been successfully synthesized in experiments. It is meaningful to explore systematically the effect of the electron or hole doping on the thermoelectric properties of the semimetal Cd3As2.
In this work, we perform first principles computations on the electronic states and thermoelectric properties of the topological semimetal Cd3As2. It is found that the electronic structure of Cd3As2 can be described correctly by a generalized gradient approximation after the spin–orbit coupling is considered. Based on the Boltzmann transport theory, we calculated the electrical conductivity, thermal conductivity, Seebeck coefficient, and figure of merit of the semimetal. The calculated thermoelectric properties of the pristine Cd3As2 match well with the experimental results. Very interestingly, the ZT value of the semimetal is found to be much improved by both electron and hole doping, especially the latter. For the n-type Cd3As2, the optimal doping level is about 1 × 1020 cm−3, leading to the ZT increasing to about 0.5 at T = 700 K, which is much larger than the ZT (0.15) of the pristine Cd3As2. In contrast, for the p-type Cd3As2, the maximal value of the Seebeck coefficient shifts towards the low temperature region and the S value in the hole-doping region is much larger than the one in the electron-doping region, resulting in an optimum ZT of about 0.5 at 500 K for the p-type Cd3As2. Our work provides new paths towards high thermoelectric performance in topological Dirac semimetals.
II. Methods and models
The calculations were performed using the general potential linearized augmented plane-wave (LAPW) method18 as implemented in the WIEN2K code.19 Spin-orbital coupling is included as a second vibrational step using scalar-relativistic eigenfunctions as the basis after the initial calculation is converged to self-consistency. The convergence of the calculations regarding the size of the basis set is achieved using an RMTKmax value of 7, where RMT is the smallest atomic sphere radius in the unit cell and Kmax is the magnitude of the largest K wave vector inside the first Brillouin zone. Exchange and correlation effects are accounted for using the generalized gradient approximation Perdew–Burke–Ernzerhof (PBE) functional.20 Considering the possible underestimation of the band gap by PBE, we do the calculation based on the modified Becke–Johnson (mBJ) potential,21 which has been proved to be a good correction to the exchange and correlation functional for electronic structure calculations of thermoelectric systems.22–24 From the calculated band structure, the thermoelectric transport tensors are evaluated using the semi-classical Boltzmann kinetic transport theory within the constant relaxation time approximation (CSTA) and the rigid band approach as implemented within the BoltzTraP program.25 The constant relaxation time is given as a standard electron–phonon model,22,26 the parameter of which is given by fitting the experimental data. This method has been successfully used to describe the transport coefficients of a wide range of thermoelectric materials.27–32III. Results and discussion
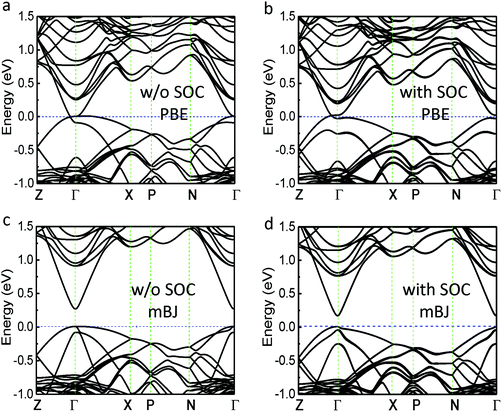
Cd3As2 has a distorted superstructure of the antifluorite (M2X) structure type with an I41/acd space group.5 It can be viewed as a tetragonally-distorted anti-fluorite structure with 1/4 M sites of vacancies and contains 80 atoms per unit cell. The calculated electronic band structures without and with spin–orbit coupling (SOC) based on the PBE potentials are shown in Fig. 1(a) and (b), respectively. As shown in Fig. 1(a), the conduction and valence bands of Cd3As2 are degenerate at the Γ point without the SOC. Similar to most semiconductors with antifluorite orzinc-blende structures, the low energy electronic properties of Cd3As2 are mostly determined by the Cd 5s states (conduction bands) and the As 4p states (valence bands). When the SOC is considered, the bands are inverted around the Γ point with the s states lower than the p states, which is an important sign of the nontrivial topology appearance. In contrast to ordinary topological insulators, no global band gap opens in the Cd3As2 even after the SOC is taken into account.5,8 Instead the bands cross around the Γ point along the Γ–Z direction, exactly at the EF, due to the protection of the C4v symmetry with respect to the kz axis. This unique band characteristic, together with the time-reversal and space inversion symmetries, gives rise to a 3D massless Dirac semimetal property of the system, in good agreement with the results of ref. 5 and 8. Since a material’s thermoelectric properties strongly depend on the band structure,25 and the mBJ potential was proved to be an excellent method for calculating the electric states of the thermoelectric systems,22–24 we did the calculation for Cd3As2 based on the mBJ potential. The electronic band structures without and with SOC based on the mBJ potential are given in Fig. 1(c) and (d), respectively. A global gap of 160 meV is opened at the Γ point in Fig. 1(d), which is inconsistent with the experimental data,8,9 indicating that the mBJ potential cannot describe well the electronic structure of 3D Dirac semimetals and may even produce a wrong result. This observation might be explained by the fact that the mBJ potential was designed to reproduce the shape of the exact exchange optimized effective potential of atoms.21,22,24 Because the mBJ potential leads to an increase of the gap values, the conduction bands and valance bands are separated too much, making the Dirac point disappear. It can be expected that if the gap opened by the mBJ in Fig. 1(c) is not very big and the band inversion can still be induced by the SOC interaction, the 3D Dirac semimetal behavior will remain in the system. The reason for this is that the mBJ potential does not break the C4v symmetry in the system.Since the electronic structure of Cd3As2 is described correctly with the PBE functional, the thermoelectric properties of Cd3As2 were investigated using the Boltzmann transport theory based on the band structures obtained with the PBE functional (Fig. 1(b)). In experiments, the fabricated intrinsic Cd3As2 is an n-type material with an electron carrier concentration of about n = 1 × 1019 cm−3,11,12 also called pristine Cd3As2 in this work. Thus, we first calculated the thermoelectric properties of Cd3As2 at this electron carrier concentration. Within the framework of the Boltzmann transport theory, the scattering time relaxation (τ) is usually adopted approximately as a constant, which is associated with the behavior of the electrical conductivity (σ), thermal conductivity from electronic contributions (ke), and furthermore the ZT of the system.25 The relaxation time generally depends on both the charge carrier concentration (n) and the temperature (T). We here employed a standard electron–phonon dependence on T and n for τ, namely, 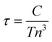 ,22,26 where C is a constant and can be determined by comparing it to experimental data. For this pristine Cd3As2 sample (with n = 1 × 1019 cm−3), the experimental electrical conductivity is about 67 S cm−1 at 300 K,11,12 which together with the ratio of σ/τ which was obtained from the Boltzmann transport theory gives a value of C of about 5 × 10−6 s K cm.
,22,26 where C is a constant and can be determined by comparing it to experimental data. For this pristine Cd3As2 sample (with n = 1 × 1019 cm−3), the experimental electrical conductivity is about 67 S cm−1 at 300 K,11,12 which together with the ratio of σ/τ which was obtained from the Boltzmann transport theory gives a value of C of about 5 × 10−6 s K cm.
The calculated electrical resistivity of the pristine Cd3As2 sample with respect to the temperature is plotted in Fig. 2(a). It shows metallic behavior at low temperatures, which is in good agreement with the experiments, indicating that the standard electron–phonon model is suitable to describe the mechanism of electron–phonon scattering in Cd3As2. Note that since the experimental data of Cd3As2 ranged from 0 K to 380 K, we plotted the thermoelectric properties obtained in our calculations also in this temperature range, as shown in Fig. 2, to compare with the experimental results directly. With the constant relaxation time approximation, the Seebeck coefficient is independent of τ. The obtained Seebeck coefficient of the Cd3As2 sample also matches the experimental data very well (Fig. 2(b)), meaning that the semiclassical Boltzmann transport theory can be adopted to describe the thermoelectric transport properties of Cd3As2. The calculated electron thermal conductivity (κe), plotted in Fig. 2(c), increases with temperature. To evaluate the ZT of this Cd3As2 sample, the lattice thermal conductivity (κL) is also required, which is obtained by subtracting κe from the total thermal conductivity (κtot) provided from experiments.11 We find that the obtained κL follows a classic A/T dependence with A = 245 W m−1, as shown in Fig. 2(c). At high temperatures, κL decreases, giving rise to κe contributing more to the total thermal conductivity. Based on the obtained σ, S, and κtot, the thermoelectric figure of merit can be estimated through the formula ZT = S2σT/κtot (Fig. 2(d)). The trend of the calculated ZT, especially in the low temperature region, is in good agreement with the experimental results.11,12 The maximum of the calculated ZT is about 0.2 at 400 K, which is also very close to the experimental values.11,12 These good agreements indicate that the Boltzmann transport theory with a constant relaxation time approximation can describe very well the thermoelectric transport properties of Dirac semimetals.
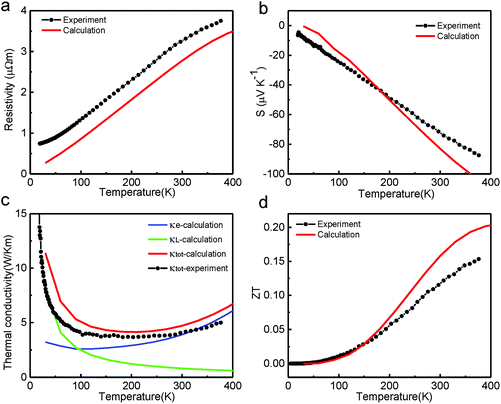 | ||
| Fig. 2 (a)–(d) Calculated temperature-dependent resistivity, Seebeck coefficient (S), thermal conductivity, and figure of merit (ZT) of Cd3As2, compared with the experimental data from ref. 11. | ||
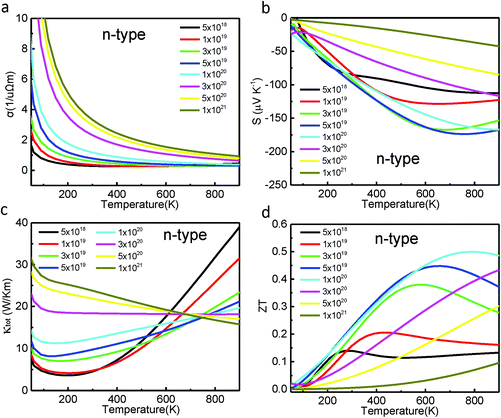
To investigate the thermoelectric properties of Cd3As2 with different electron carrier concentrations, the rigid-band approach was employed. Fig. 3 shows the evolution of the electrical conductivity with respect to the temperature for various electron carrier concentrations of interest. At a fixed temperature, the electrical conductivity increases drastically with the carrier concentration. When the concentration is n = 1 × 1021 cm−3, the electrical conductivity increases to 3.81/μΩ m at 300 K, which is much higher than that of the pristine Cd3As2 (2.62/μΩ m at 300 K). The calculated Seebeck coefficient S (Fig. 3(b)) has, however, different trends as a function of the temperature for various electron carrier concentrations. When the electron concentration is low (n ≤ 5 × 1019 cm−3), the absolute value of the calculated S (|S|) increases with the temperature and then decreases. The maximal value of |S| increases with concentration and its temperature position shifts up. For high electron concentrations (n > 1 × 1020 cm−3), the calculated |S| increases almost linearly with temperature in the range below 900 K, like a metal.1 As a consequence, for the n-type doping, the maximum of the calculated |S| value is about 170 µV K−1 with n = 5 × 1019 cm−3 at 700 K. Combining the electron thermal conductivity and lattice thermal conductivity, we plotted the total thermal conductivity with respect to the temperature at various concentrations in Fig. 3(c). Since the κL value decreases drastically with temperature (κL = A/T), the trend of κtot is determined by the κe for temperatures larger than 200 K. With the obtained σ, S, and κtot, the figure of merit of the material at various electron concentrations can be calculated, which is presented in Fig. 3(d). Obviously, the ZT shares a similar tendency to |S| as a function of concentration (Fig. 3(b)) to a certain extent. The maximum optimum ZT of Cd3As2 is found to be about 0.5 at T = 700 K with n = 1 × 1020 cm−3. This carrier concentration (n = 1 × 1020 cm−3) is hopefully achieved in experiments with current advanced technologies.11,16,33 This predicted ZT value (0.5) of Cd3As2 with electron doping is much larger than the maximum ZT (0.15) achieved in the experiments for the pristine samples.11,12
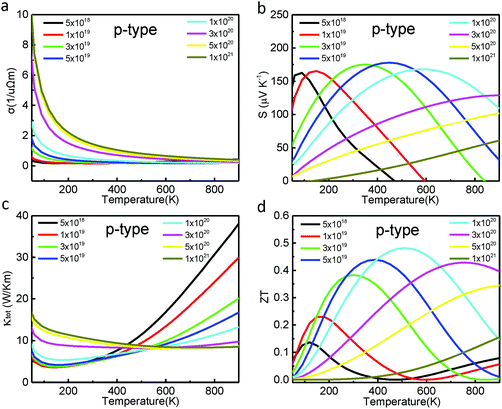
The thermoelectric behavior of Cd3As2 with hole doping is also explored since electron and hole doping are both easily carried out in semimetals in experiments.16,17 As shown in Fig. 4(a) and (c), the trends of both electronic conductivity and thermal conductivity of the p-type Cd3As2 as functions of temperature and concentration are similar to those of the n-type Cd3As2. The magnitudes of σ and κtot in the p-type Cd3As2 are, however, usually lower than those of the n-type Cd3As2 at a fixed temperature or concentration, which is associated with the band structures. The S curves in the p-type Cd3As2 (Fig. 4(b)) are very different from those of the n-type Cd3As2 (Fig. 3(b)). Fig. 4(b) shows that the maximal values of S at various hole concentrations are located at lower temperatures than those of the n-type Cd3As2 and the S absolute values in the hole-doping region are much larger than the corresponding ones in the electron-doping region. As a result, the ZT of the p-type Cd3As2 is enhanced greatly for the middle temperature region (Fig. 4(d)), compared to that of the n-type sample. For the p-type Cd3As2, an optimum ZT of about 0.5 can be acquired at 500 K with a hole doping concentration of p = 1 × 1020 cm−3, as shown in Fig. 4(d).
Because the concentrations of Cd3As2 are usually tuned by the gate voltage,16,17 it is significant to report the thermoelectric properties with respect to chemical potential at various temperatures. Thus, we plot the σ, S, κtot, and ZT in terms of chemical potential at various temperatures as Fig. S1(a)–(d), respectively, in the ESI.† At the same temperature, the maximum value of σ with electron doping is larger than that with hole doping and the σ of both n-type and p-type Cd3As2 decreases with temperature (Fig. S1(a)†), which is consistent with the trends in Fig. 3(a) and 4(a). For the Seebeck coefficient, it is interesting to find that its maximum values of hole doping at different temperatures all occur at about 0.1 eV below the EF, where the band dispersion is weak (Fig. 1(b)). Meanwhile for the electron doping, the S maximum values all occur at about 0.25 eV above the EF due to the degenerated bands around this energy (Fig. 1(b)). For most temperatures, the S maximum values of p-type Cd3As2 are larger than those of n-type Cd3As2, resulting in a larger ZT in p-type Cd3As2, which is also in agreement with the trends obtained from Fig. 3(d) and 4(d). The reason why the thermoelectric behavior of the p-type Cd3As2 is superior to that of the n-type Cd3As2 can be ascribed to the band dispersions around the EF. The relation between temperature and Seebeck coefficient can be seen using relatively simple models of electron transport. For metals or degenerate semiconductors, the Seebeck coefficient is approximately given by:1
IV. Conclusions
In summary, we performed first principles computations on the electronic structure and thermoelectric behavior of the topological semimetal Cd3As2 and found that the electronic structure of Cd3As2 can be described correctly by the generalized gradient approximation with spin–orbit coupling. Based on the Boltzmann transport theory, the electrical conductivity, thermal conductivity, Seebeck coefficient, and figure of merit of the system were calculated. The optimum ZT of Cd3As2 with electron-doping was found to be about 0.5 at T = 700 K with n = 1 × 1020 cm−3, while for the p-type Cd3As2, the maximal value of the Seebeck coefficient as a function of temperature increases with the increase of the hole doping concentration and its position shifts drastically towards the lower temperature region compared to that of the n-type Cd3As2, leading to a high ZT (0.5) of the p-type Cd3As2 being achieved at low temperature (500 K). Our work provides a new avenue towards high-performance thermoelectric materials based on topological Dirac semimetals.Acknowledgements
This work was supported by the National Natural Science Foundation of China with Grant No. 11574051, the Natural Science Foundation of Shanghai with Grant No. 14ZR1403400, and the Fudan High-end Computing Center.References
- J. P. Heremans, V. Jovovic, E. S. Toberer, A. Saramat, K. Kurosaki, A. Charoenphakdee, S. Yamanaka and G. J. Snyder, Science, 2008, 321, 554 CrossRef CAS PubMed
.
- G. J. Snyder and E. S. Toberer, Nat. Mater., 2008, 7, 105 CrossRef CAS PubMed
.
- I. Terasaki, Y. Sasago and K. Uchinokura, Phys. Rev. B: Condens. Matter, 1997, 56, R12685 CrossRef CAS
.
- S. Hebert, S. Lambert, D. Pelloquin and A. Maignan, Phys. Rev. B: Condens. Matter, 2001, 64, 172101 CrossRef
.
- Z. Wang, H. Weng, Q. Wu, X. Dai and Z. Fang, Phys. Rev. B: Condens. Matter, 2013, 88, 125427 CrossRef
.
- Z. K. Liu, J. Jiang, B. Zhou, Z. J. Wang, Y. Zhang, H. M. Weng, D. Prabhakaran, S. K. Mo, H. Peng, P. Dudin, T. Kim, M. Hoesch, Z. Fang, X. Dai, Z.-X. Shen, D. L. Feng, Z. Hussain and Y. L. Chen, Nat. Mater., 2014, 13, 677 CrossRef CAS PubMed
.
- S. Jeon, B. B. Zhou, A. Gyenis, B. E. Feldman, I. Kimchi, A. C. Potter, Q. D. Gibson, R. J. Cava, A. Vishwanath and A. Yazdani, Nat. Mater., 2014, 13, 851 CrossRef CAS PubMed
.
- M. Neupane, S.-Y. Xu, R. Sankar, N. Alidoust, G. Bian, C. Liu, I. Belopolski, T.-R. Chang, H.-T. Jeng, H. Lin, A. Bansil, F. Chou and M. Z. Hasan, Nat. Commun., 2014, 5, 3786 CAS
.
- T. Liang, Q. D. Gibson, M. N. Ali, M. Liu, R. J. Cava and N. P. Ong, Nat. Mater., 2015, 14, 280 CrossRef CAS PubMed
.
- Y. Pei, X. Shi, A. LaLonde, H. Wang, L. Chen and J. Snyder, Nature, 2011, 473, 66 CrossRef CAS PubMed
.
- C. Zhang, T. Zhou, S. Liang, J. Cao, X. Yuan, Y. Liu, Y. Shen, Q. Wang, J. Zhao, Z. Yang and F. Xiu, Chin. Phys. B, 2016, 25, 017202 CrossRef
.
- A. Pariari, N. Khan and P. Mandal, 2015, arXiv:1502.02264.
- I. Gierz, C. Riedl, U. Starke, C. R. Ast and K. Kern, Nano Lett., 2008, 8, 4603 CrossRef CAS PubMed
.
- T. Hosseini, N. Yavarishad, J. Alward, N. Kouklin and M. Gajdardziska-Josifovska, Adv. Electron. Mater., 2016, 2, 1500319 CrossRef
.
- A. Pariari, N. Khan and P. Mandal, 2015, arXiv:1508.02286.
- Y. Liu, C. Zhang, X. Yuan, T. Lei, C. Wang, D. D. Sante, A. Narayan, L. He, S. Picozzi, S. Sanvito, R. Che and F. Xiu, NPG Asia Mater., 2015, 7, e221 CrossRef CAS
.
- C. Li, J. Li, L. Wang, L. Zhang, J. Zhang, D. Yu and Z. Liao, ACS Nano, 2016, 10, 6020 CrossRef CAS PubMed
.
-
D. J. Singh and L. Nordstrom, Planewaves, Pseudopotentials, and the LAPW Method, Springer, Berlin, 2nd edn, 2006 Search PubMed
.
-
P. Blaha, K. Schwarz, G. Madsen, D. Kvasnicka and J. Luitz, WIEN2k, An Augmented Plane Wave+Local Orbitals Program for Calculating Crystal Properties, Tech. Univ.Wien, Austria, 2001 Search PubMed
.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed
.
- F. Tran and P. Blaha, Phys. Rev. Lett., 2009, 102, 226401 CrossRef PubMed
.
- P. Boulet and M. C. Record, J. Chem. Phys., 2011, 135, 234702 CrossRef PubMed
.
- J. J. Pulikkotil, D. J. Singh, S. Auluck, M. Saravanan, D. K. Misra, A. Dhar and R. C. Budhani, Phys. Rev. B: Condens. Matter, 2012, 86, 155204 CrossRef
.
- H. Y. Lv, W. J. Lu, D. F. Shao and Y. P. Sun, Phys. Rev. B: Condens. Matter, 2014, 90, 085433 CrossRef
.
- G. K. H. Madsen and D. J. Singh, Comput. Phys. Commun., 2006, 175, 67 CrossRef CAS
.
- K. P. Ong, D. J. Singh and P. Wu, Phys. Rev. B: Condens. Matter, 2011, 83, 115110 CrossRef
.
- D. Parker and D. J. Singh, Phys. Rev. B: Condens. Matter, 2010, 82, 035204 CrossRef
.
- D. J. Singh and I. I. Mazin, Phys. Rev. B: Condens. Matter, 1997, 56, R1650 CrossRef CAS
.
- Y. Yokomizo and J. Nakamura, Appl. Phys. Lett., 2013, 103, 113901 CrossRef
.
- G. K. H. Madsen, K. Schwarz, P. Blaha and D. J. Singh, Phys. Rev. B: Condens. Matter, 2003, 68, 125212 CrossRef
.
- Y. Saeed, N. Singh and U. Schwingenschlogl, Appl. Phys. Lett., 2014, 104, 033105 CrossRef
.
- K. Tyagi, B. Gahtori, S. Bathula, S. Auluck and A. Dhar, Appl. Phys. Lett., 2014, 105, 173905 CrossRef
.
- E. Zhang, Y. Liu, W. Wang, C. Zhang, P. Zhou, Z. G. Chen, J. Zou and F. Xiu, ACS Nano, 2015, 9, 8843 CrossRef CAS PubMed
.
- D. J. Singh, Phys. Rev. B: Condens. Matter, 2010, 81, 195217 CrossRef
.
- D. J. Singh, Phys. Rev. B: Condens. Matter, 2007, 76, 085110 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available: Calculated electrical conductivity (σ), Seebeck coefficient (S), thermal conductivity (κ), and figure of merit (ZT) of n/p-type Cd3As2 with respect to the chemical potential at different temperatures and calculated densities of states for the Cd3As2 crystal. See DOI: 10.1039/C6QI00383D |
| This journal is © the Partner Organisations 2016 |