Reduced graphene oxide–transition metal hybrids as p-type semiconductors for acetaldehyde sensing†
Yusuke
Murashima
a,
Mohammad Razaul
Karim
ab,
Ryo
Furue
a,
Takeshi
Matsui
a,
Hiroshi
Takehira
a,
Kosuke
Wakata
a,
Kei
Toda
a,
Ryo
Ohtani
a,
Masaaki
Nakamura
a and
Shinya
Hayami
*ac
aGraduate School of Science and Technology, Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto, 860-8555, Japan. E-mail: hayami@sci.kumamoto-u.ac.jp; Fax: +81-96-342-3469; Tel: +81-96-342-3469
bDepartment of Chemistry, School of Physical Sciences, Shahjalal University of Science & Technology, Sylhet-3114, Bangladesh
cInstitute of Pulsed Power Science (IPPS), Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto, 860-8555, Japan
First published on 11th April 2016
Abstract
Acetaldehyde gas sensing using hybrids of reduced graphene oxide (rGO) and transition metal elements, rGO–M (M = oxide/hydroxide of Mn, Fe, Co and Ni) has been investigated. Thin films of GO, rGO and rGO–M on conductive glass were deposited through simple and affordable techniques and characterized using Raman spectroscopy, powder X-ray diffraction patterns, field emission scanning electron microscopy and electrical conductivity measurements. Concentration dependent resistances during the oxidation of acetaldehyde gas in air (10 to 50%) were investigated by using a sensing probe devised from rGO, GO–M and the rGO–M hybrids, of which rGO–Ni exhibited the maximum sensitivity. In rGO–Ni, in combination with rGO the NiOOH precursor can capture electrons (generated from acetaldehyde oxidation) through the holes, while indicating rGO–Ni to be a p-type semiconductor. This report implies the possibility of developing inexpensive graphene based p-type semiconductors for sensing other gases as well.
Introduction
Carbon allotropes can function as excellent carriers for scaffolding semiconductor components in sensing devices, especially where an electron transfer process plays a principal role in the detection of a target analyte.1 In this respect, though the gas sensing properties of some hybrids of metal oxide semiconductors including ZnO, SnO2 and TiO2 with carbon nanotubes have been reported, attempts for employing graphene (G) or reduced graphene oxide (rGO) as a conductive dispersion phase are only a recent concept.2 But, significant evidence exists of the possibility for rGO to support metals/metal oxides/hydroxides or similar without interrupting their functionalities.3 The support of GO/rGO materials for transition metal (TM) ions or semiconductor nanoparticles is significant, as the metal precursor usually becomes embedded on the carbon framework through some two dimensional arrangement, which results in the optimum accommodation of the metal component on a conductive carbon support with increased functionality. Though it is possible to detect various hazardous and inflammable gases including H2, CO2 and NOX in air samples using GO or rGO based hybrids, there exists only a few reports for sensing volatile organic compounds like acetaldehyde.4Acetaldehyde is a highly toxic material. It is used majorly in industry for the synthesis of polymers, organic compounds and resins. Of the aldehydes, acetaldehyde is the commonest and is a forbidden hazardous contaminant in drinking water. It is a potential cancer causing agent and genotoxicant as it can interact with DNA through cellular metabolism.5 Therefore, both the qualitative and quantitative determination of acetaldehyde especially in gaseous samples is necessary for ensuring human safety. Herein, we report the sensing of acetaldehyde gas by using rGO–M; (M = oxide/hydroxide of Mn, Fe, Co and Ni). Nowadays, various methods including colorimetric, chromatographic, spectrometric, quartz crystal microbalance and electrochemical detection processes are used for sensing acetaldehyde.6 Though each process possesses its own advantages and disadvantages, electrochemical methods are the commonest due to the simple and affordable techniques, a wide range of detection limits and low fabrication costs.7 We have employed concentration dependent resistivity for sensing acetaldehyde by using a glass supported thin sheet of rGO–M as a sensing probe.
rGO has the inherent possibility to be a dispersing phase and electron mediator in sensors.8 Gas sensors based on this 2D carbon allotrope theoretically hold some advantages over sensors devised from metal oxide semiconductors. For example, a metal oxide semiconductor gas sensor needs a high operational temperature (typically 300–450 °C), but carbon based hybrid sensors can function at room temperature.9 In addition, as the sensitivity of a probe depends on the active material's surface area, porosity, morphology and conductivity, using some conductive and chemically functionalized platform to scaffold TM particles with catalytic activity towards the sensing reaction can allow enhanced performance to be achieved. The catalytic component can catalyze the very selective electron transfer reaction of the analyte,10 whereas, the faster electron transfer process from the reaction site to the electrode is supported by the conductive carbon matrices.11 The increase in surface area of the TM particles during their dispersion in the conductive matrices is an additional advantage.
Considering all these facts, graphene might be an excellent candidate for sensor fabrication. But, as pure graphene is highly inactive, we employed negatively charged GO for the formation of a GO–M non conductor hybrid, which after reduction is converted into a conductive rGO–M hybrid.12 The hybrids could successfully detect acetaldehyde in air samples with the maximum sensitivity exhibited by the rGO–Ni hybrid.
Experimental
Synthesis of GO
GO was synthesized from slight modification to Hummers method.13 In short, graphite powder was oxidized by NaNO3, H2SO4 and KMnO4 at 95 °C. It was diluted by water and H2O2 (30%). The precipitate was washed repeatedly with 10% HCl solution and water. It was then dried at 70 °C for 24 hours. This dispersion was centrifuged at 4000 rpm for 1 h and the supernatant liquid was called graphene oxide (GO) dispersion.Fabrication of glass supported thin film of rGO and rGO–M hybrids as the sensing probe
25 mL of GO dispersion (0.1 g L−1) was mixed with each of 100 μmol Mn(CH3COO)2·4H2O, FeCl3·6H2O, CoCl2·6H2O, and NiCl2·6H2O. The black solutions were vigorously stirred at 25 °C for 24 h to obtain GO–M hybrids. 60 μL of a GO–M hybrid was dropped on the surface of an ITO glass substrate (6 mm × 15 mm) followed by drying at 80 °C. The high temperature resulted in the thermal reduction of GO–M into a rGO–M hybrid. The pouring and drying was accomplished successively three times to obtain a rigid and stable rGO–M coating on glass. As, the substrates prepared with GO and GO–Mn coating were stable to thermal reduction, they were annealed in hydrazine vapour at 70 °C for 24 h. The brown color of the GO–M hybrids turned into black on the substrate after being reduced to rGO–M hybrids. Finally after drying under vacuum, two electrodes (4 mm apart) were connected to the thin rGO–M films with gold paste.Physical measurements
A micro Raman spectrometer (NRS-3100, JASCO, Japan) with a 532 nm excitation source at room temperature was used to collect the Raman spectra. The powder X-ray diffraction (XRD) patterns were recorded on a Rigaku X-ray diffractometer (RAD-2A with a 2.0 kW CuKα X-ray). The field emission scanning electron microscopy (FE-SEM, JEOL JSM-7600F with accelerating voltage of 1.0 kV) was used to observe the morphologies of the substrates.Gas sensing
For acetaldehyde gas sensing, the thin glass supported rGO–M probes (devised through the process described above) were connected to a potentiostat (ALS/DY2323 BI-POTENTIOSTAT, BAS Inc., Japan). The probe was then fitted with an experimental quartz made sample chamber that had an inlet and outlet tube connected to two air tight flasks containing acetaldehyde solution and pure air. The flow of pure air or a mixture of acetaldehyde and air with a varied ratio could be maintained by fixing their relative amounts through control valves. Firstly, at room temperature with 40% relative humidity (RH), pure air flow was continued to remove all unwanted contaminants from the sample chamber. The resistance scanned with respect to time was monitored. The air flow was stopped until a stable resistance (Ra) was displayed. The chamber was then injected with a known amount of acetaldehyde through the inlet tube and the resistance in acetone gas (Rg) was recorded. The sensitivity was calculated from the ratio Rg/Ra. The chamber was purged again until the recovery of the Ra value. Ra had an almost fixed value, whereas the Rg value varied with respect to the variation of acetaldehyde gas concentration. Various sensitivity (Rg/Ra) values were determined by repeating the methods sequentially.14Results and discussion
The thin GO, rGO, GO–M and rGO–M layers deposited on glass substrates were characterized using Raman spectroscopy, powder X-ray diffraction patterns and FE-SEM. Optical photographs in Fig. 1a show that the brown colored coating on the glass substrates turns black due to the reduction of GO into rGO. Fig. 1b represents the S spectra of GO, GO–Ni and rGO–Ni hybrids.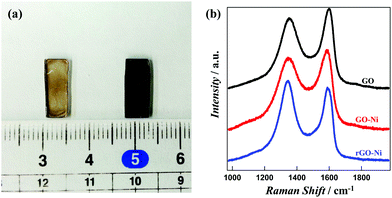 | ||
| Fig. 1 (a) Photograph of glass supported films for GO (left) and rGO (right). (b) Raman spectra of GO, GO–Ni and rGO–Ni. | ||
The relative domain size of the sp2 and sp3 carbon sites confirms the successful conversion of GO into rGO and the hybrids. The G (1580 cm−1) and D (1350 cm−1) bands originate from the E2g phonon corresponding to sp2 carbon atoms and the κ-point phonon of A1g symmetry for the breathing mode of sp3 carbon, respectively. The peak ratios (ID/IG) are 1.09, 0.906 and 0.884 for GO, GO–Ni and rGO–Ni hybrids, respectively. The ID/IG value is inversely proportional to the extent of the sp2 domain.15 The gradual decrease in the ID/IG values during the conversion of GO → GO–Ni → rGO–Ni therefore demonstrates a gradual increase in sp2 domain size. In fact, the coulombic association of a positively charged metal ion with negatively charged GO leads to the polarization of the electron cloud of GO towards the metal ions. After completion of the reduction the increase in sp2 character confirms the conversion of GO into rGO.16 The other hybrids demonstrate almost similar trends in their Raman spectra (Fig. S1†). In addition to Raman spectroscopic analysis, IR spectra depicting the vibrational modes of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–OH are presented in Fig. S2.† Due to the reduction of GO into rGO, the intensities of the peaks associated to these groups decrease significantly.
O and C–OH are presented in Fig. S2.† Due to the reduction of GO into rGO, the intensities of the peaks associated to these groups decrease significantly.
Fig. 2 represents the PXRD patterns of GO, rGO, GO–M and rGO–M hybrids. GO exhibits a characteristic peak at 12.4° (2θ), indicating an interlayer distance of about 7.13 nm. This characteristic d spacing value indicates the existence of intercalated epoxy oxygens extended outward from the GO basal plane and is consistent with previous reports.17 In rGO, this peak vanishes due to the decomposition of the epoxy groups. The presence of metal precursor in GO–Ni, rGO–Ni, GO–Co and rGO–Co is confirmed from the existence of sharp peaks in the respective PXRD patterns. GO–Ni exhibits characteristic peaks at 15.7°, 18.3° and 30.4° with respective interlayer distances of 0.56, 0.48 and 0.29 nm. In rGO–Ni the peak positions at 13.5° and 23.5° correspond to layer separations of 0.66 and 0.38 nm. After reduction, the surface separation is wider than GO–Ni, which is supposed to result from the formation of metal oxide nanoclusters as discussed in our previous report.12 In fact, for rGO–Ni the observation indicates the existence of γ-Ni(OOH).18 GO–Co exhibits characteristic peaks at 5.7°, 8.3° and 30.5°, while rGO–Co exhibits two peaks at 15.9° and 20.6°. This observation indicates the formation of cobalt oxide.19 Though the interlayer distance in GO–Co is similar to that in GO–Ni, the layer separations in rGO–Co of 0.56 and 0.43 nm indicate the existence of Co3O4 in rGO–Co.20 In contrast to GO–Ni and GO–Co, GO–Mn does not exhibit any peaks in its PXRD pattern. Mn therefore is expected to exist in an amorphous state in GO. After reduction, rGO–Mn shows characteristic peaks at 10.3° with a layer distance of 0.86 nm which indicates the existence of α-MnO2 in the hybrid.21 Instead of any sharp peaks, GO–Fe exhibits a characteristic broad peak at 12.0°. In rGO–Fe, the peak vanishes as the structure changes to an inhomogeneous state during reduction. We checked the morphology by FE-SEM to establish this evidence.
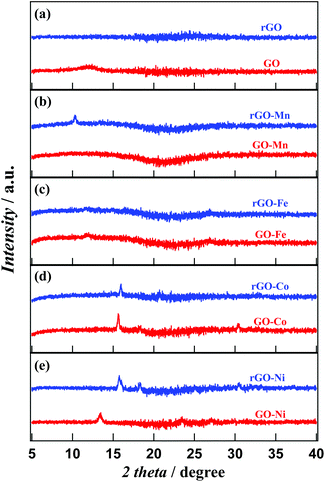 | ||
| Fig. 2 PXRD patterns of (a) GO and rGO, (b) GO–Mn and rGO–Mn, (c) GO–Fe and rGO–Fe, (d) GO–Co and rGO–Co and (e) GO–Ni and rGO–Ni. | ||
Fig. 3 represents the FE-SEM images of GO, rGO, GO–M and rGO–M hybrids. The surface morphology clearly indicates that GO possesses a transparent layered structure, whereas rGO is almost amorphous (Fig. 3a and b). During reduction, the removal of oxygen atoms results in the loss of polarity and the destruction of the layered structure of GO.
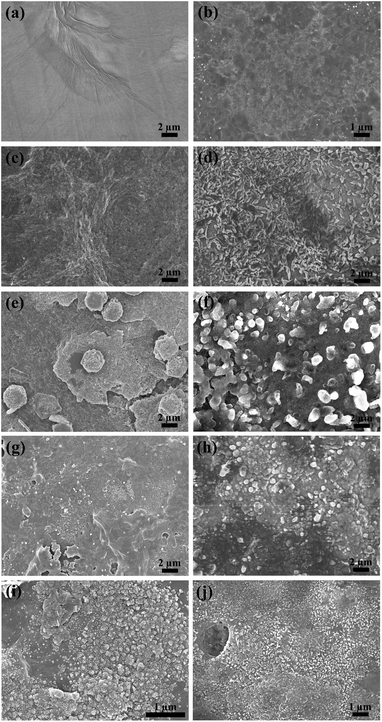 | ||
| Fig. 3 FE-SEM images of (a) GO, (b) rGO, (c) GO–Mn, (d) rGO–Mn, (e) GO–Fe, (f) rGO–Fe, (g) GO–Co, (h) rGO–Co, (i) GO–Ni and (j) rGO–Ni. | ||
Except for GO–Mn (Fig. 3c and d), the presence of TM precursors in all the other hybrids is clear from the appearance of denser spots (Fig. 3e–i). The particle sizes in GO–Fe, GO–Co and GO–Ni are about 3 μm, 200–300 nm and 100–200 nm, respectively (Fig. 3e, g and i). After reduction the particle sizes of the metal precursors in rGO–Fe, rGO–Co and rGO–Ni became <1 μm, 1 μm–500 nm and 300–200 nm, respectively. Though the asperity in GO–Mn surface indicates the absence of any particles, the flake-like structures (1 μm) after reduction indicate the presence of Mn in rGO–Mn. The absence of any peaks in the PXRD data (Fig. 2) of GO–Mn also complies with its amorphous structure revealed by the FE-SEM images. The fabrication of hybrid layers on the glass substrate was also confirmed from the height profiles of the layers. Fig. S3 in the ESI† shows a cross section of the substrates. Before reduction, the thicknesses of GO, GO–Mn, GO–Fe, GO–Co and GO–Ni were 0.834 μm, 1.350 μm, 1.041 μm, 1.153 μm and 0.863 μm, respectively. However, after reduction the respective thicknesses changed to 0.881 μm, 1.388 μm, 1.238 μm, 1.603 μm and 1.350 μm. Therefore, reduction has an insignificant effect on the thicknesses of the deposited films.
SEM-EDX analysis of the samples in Fig. S4† confirms the presence of heavy metal precursors on the GO and rGO structures. The mapping indicates a random dispersion of the metal/metal oxide clusters throughout the nanosheets.
Fig. 4 represents the current vs. voltage plots (I–V curves) for GO, GO–M, rGO and rGO–M hybrids on the glass substrate. For each sample, we measured the conductivity with respect to both the negative and positive biasing. GO and all the GO–M hybrids exhibit insulation properties irrespective of the magnitude of the applied voltage. The current for rGO and rGO–M hybrids increases gradually with biasing the voltage (forward/reverse). At 1.0 V, the conductivities are around 160, 100, 50, 30 and 220 μA for rGO, rGO–Mn, rGO–Fe, rGO–Co and rGO–Ni, respectively. The resistance of rGO–Ni is the lowest, while the resistances of rGO–Mn, rGO–Co and rGO–Fe are higher than that of pristine rGO. For rGO–Mn, rGO–Co and rGO–Fe, we propose that some of the oxygen functional groups remain unchanged even after the reduction of GO. These unreduced oxygenous sites block the conduction path.22,23 In contrast, nickel oxide/hydroxide in the rGO–Ni hybrids are expected to function as a p-type semiconductor while being anchored on rGO nanosheets. The surge in the number of holes increases which results in the increased conductivity of rGO–Ni, compared with the other hybrids. Insulator GO and GO–M hybrids show a current voltage plot similar to the GO–Ni hybrid as indicated by the black line in Fig. 4.
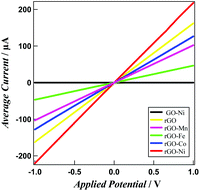 | ||
| Fig. 4 Averaged current vs. applied voltage plots for GO–Ni (black), rGO (yellow), rGO–Mn (purple), rGO–Fe (green), rGO–Co (blue) and rGO–Ni (red). | ||
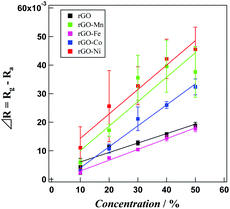
Fig. 5 presents the time dependent Rg/Ra values for sensing acetaldehyde gas within the concentration range of 10–50% in air, using various rGO–M hybrids. The sensitivity of the rGO probe remains almost constant irrespective of the concentration of acetaldehyde. Except for rGO–Fe, the other rGO–M hybrids exhibit higher sensitivity compared with pristine rGO. In fact, for all the samples the Rg/Ra values increase gradually. At 30% acetaldehyde content, the Rg/Ra values for rGO, rGO–Mn, rGO–Fe, rGO–Co and rGO–Ni based probes reach 1.012, 1.013, 1.028, 1.014 and 1.026, respectively. However, through a gradual increment these values increase further to 1.018, 1.039, 1.021, 1.030 and 1.043, respectively at the optimum sample concentration (50%). rGO–Fe (Fig. 5b) and rGO–Ni (Fig. 5d) based probes exhibit the lowest and highest sensitivity, respectively. For the rGO–Mn probe the sensitivity does not vary significantly beyond an acetaldehyde concentration of 40%. The sensitivity values might be affected by the gas adsorption properties of the hybrids. Therefore, to understand the extent of acetaldehyde adsorption, the response and recovery times (the time needed to reach 90% of total signal change when the sample gas mixture enters and leaves, respectively, from the cell) were monitored. Tables 1 and 2 show the average response and average recovery times for all the hybrids at various concentrations. Both the response and recovery times are shortest when the probe is fabricated using the rGO–Co hybrid, which indicates that the adsorption/desorption process of acetaldehyde on the rGO–Co hybrid takes place the fastest.
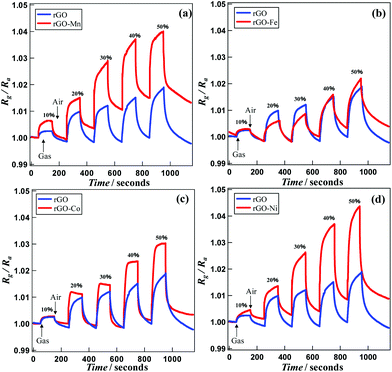 | ||
| Fig. 5 Gas sensing properties for (a) rGO and rGO–Mn, (b) rGO and rGO–Fe, (c) rGO and rGO–Co and (d) rGO and rGO–Ni. | ||
| Concentration (%) | 10% | 20% | 30% | 40% | 50% |
|---|---|---|---|---|---|
| rGO (s) | 36.5 | 42.8 | 45.9 | 52.4 | 51.2 |
| rGO–Mn (s) | 41.9 | 46.2 | 63.1 | 63.7 | 56.5 |
| rGO–Fe (s) | 40.2 | 74.3 | 71.9 | 72.7 | 76.0 |
| rGO–Co (s) | 41.7 | 9.5 | 12.8 | 23.2 | 23.2 |
| rGO–Ni (s) | 70.3 | 42.2 | 47.4 | 42.2 | 34.9 |
| Concentration (%) | 10% | 20% | 30% | 40% | 50% |
|---|---|---|---|---|---|
| rGO (s) | 56.3 | 52.0 | 49.6 | 45.8 | 50.7 |
| rGO–Mn (s) | 71.7 | 56.5 | 52.1 | 51.9 | 47.0 |
| rGO–Fe (s) | 84.6 | 70.0 | 70.5 | 72.5 | 68.8 |
| rGO–Co (s) | 50.4 | 40.1 | 28.3 | 28.8 | 28.9 |
| rGO–Ni (s) | 52.4 | 48.8 | 36.1 | 32.0 | 38.2 |
For a clear indication of the linearity between concentration and sensing responses, concentration dependent signal variations in terms of ΔR (Rg − Ra) are presented in Fig. 6. The correlation coefficient values for the dataset associated with rGO, rGO–Mn, rGO–Fe, rGO–Co and rGO–Ni are calculated to be 0.941, 0.832, 0.992, 0.985 and 0.956, respectively. This finding indicates that except for rGO–Mn, all the other samples display excellent concentration dependent signal variations, utilizable for device fabrication.
Our previous report on the coexistence of electrical conductivity and ferromagnetism in rGO–TM oxide hybrids (TM = Fe, Co and Ni) synthesized in solution phase revealed that both conductivity and magnetic properties were affected greatly by the morphology of the dispersed phase.12 Therefore, in the current case, it was expected that the state of TM and/or their oxide/hydroxide in the rGO–M hybrids might have a considerable effect on the conductivity, catalysis and extent of the acetaldehyde adsorption/desorption process. Especially, realizing the necessity for the fineness of the TM precursor during sensor fabrication, the possibility of a solid state reaction to deposit the semiconductor component on the rGO platform was taken into consideration. Herein, we succeeded in synthesizing rGO nanosheet scaffolded TM oxide/hydroxide on a glass substrate avoiding reduction in solution state. GO has negative charge, for this reason it exists in a colloidal state in polar solvent. The existence of electrostatic bonding between TM ions and negatively charged sites of GO plays the major role in the stability of the hybrids. Therefore, the formation of rGO–M hybrids is initially associated with the attachment of some primary metal ions to the partially charged oxygenated sites of GO nanosheets. These oxygenated sites, being engaged with metal ions, become stable towards reduction. However, the metal ions containing a higher amount of charge compared with the partial charge of the GO nanosheet scaffold can undergo growth by gradually attracting oxygen atoms, metal oxide molecules (produced during the reduction process) and more free metal ions. Finally, the rGO matrices can support some irregular shaped metal oxide particles.12 The SEM images of the hybrids imply the irregular shapes and sizes of the TM/TM oxide clusters dispersed on GO/rGO matrices. Though in the Fe, Co and Ni hybrids the clusters are almost spherical, in the Mn hybrids the particles look like metal filings. EDX mappings (Fig. S4†) indicate an almost homogeneous dispersion of the particles within the GO/rGO matrices. In the Fe hybrids the particle clusters seemingly possess the maximum size, with 1–2 μm diameters. The length and width of the filings in the Mn hybrids are about 1 μm and 100 nm, respectively. The particle sizes in the Co and Ni hybrids are less than 1 μm and the size of the TM/TM oxide precursors are the minimum in the rGO–Ni hybrid. Our attempt to incorporate a TM precursor on rGO for designing rGO–M hybrids as an active material for acetaldehyde sensing was considered for the possibility of meeting some requirements including: (1) in situ generation of the semiconductor component (oxide/hydroxide of TM) within rGO matrices; (2) the TM precursor might weaken the molecular bonding of oxygen (present in the air sample) at room temperature to generate an oxygen anion, which would react with an acetaldehyde molecule to oxidize, while ejecting an electron and (3) the ejected electron might combine with the holes of the semiconductor component to increase the resistance and display a signal during sensing.
The acetaldehyde sensing mechanism is associated with the generation of an oxygen anion followed by the oxidation of acetaldehyde and ejection of two electrons (eqn (1)–(3)).
| O2 (gas) → O2 (adsorbed) | (1) |
| O2 (adsorbed) + 2e− (rGO) → 2O− | (2) |
| CH3COH + O− (on GO) → CH3COOH + e− | (3) |
The oxygen molecules are adsorbed on the surface of the metallic component at the initiation step.22 Though in normal cases the collision of O2 (adsorbed) with two consecutive electrons takes place at high and low temperature, respectively, the TM present in the hybrids can catalyze the process through the interaction between its vacant ‘d’ orbital with the bonding orbital of an oxygen molecule. Due to such an interaction, the weakening of oxygen's molecular orbital could take place and a free O− ion is generated at room temperature. The presence of TM is therefore significant. In the next step, an acetaldehyde molecule is oxidized into acetic acid while reacting with the O− anion along with the ejection of a free electron translating towards the rGO conductive framework. As the resistance increases with the concentration of acetaldehyde we propose that some electron–hole recombination takes place. Therefore, the TM oxides within rGO–M hybrids behave as p-type semiconductors. The greater the concentration of acetaldehyde, the more that electron–hole recombination takes place and the electron passivity through the external circuit becomes hindered. Therefore, the resistances of the probes increase with respect to the analyte concentration. The highest sensitivity of rGO–Ni indicates that it contains the maximum number of holes. In fact the PXRD data reveals that the metallic part in the rGO–Ni hybrids exists in the form of NiOOH precursors, which is a well functioning p-type semiconductor.23 As rGO itself is a p-type semiconductor as well, in combination with NiOOH nanoparticles rGO–Ni possesses the maximum number of holes to capture the electrons generated from the oxidation of acetaldehyde. Therefore, the displayed resistance is highest for the rGO–Ni substrate compared with the other hybrids. The sensitivity of rGO–Fe is even lower than that of rGO, which suggests that rather than capturing into holes, rGO–Fe mediates the generation of the maximum number of electrons from acetaldehyde oxidation. The lower number of holes in the rGO–Fe sample is responsible for both its lower conductivity and lower electron–hole recombination.
The high surface area of rGO ensures excess adsorption sites for both the target gas (acetaldehyde) and oxygen. The electronic conductivity of rGO aids the electrons quickly spreading to the surface of the semiconductor component. In addition, rGO can effectively prevent agglomeration of metal oxide particles and metal hydroxide nanosheets. As a result, the dispersed nanoparticles have a smaller size with a high surface area, which enhances their sensing performance significantly. At the current state the Rg/Ra value for the rGO sample increases slightly (Fig. 5) during the sensitivity measurements using all the samples. We propose that the reason behind such an increment is the decomposition of some acetaldehyde molecules and some electron hole recombination at the rGO surface. rGO itself is supposed to possess some holes which are generated from the photochemical reduction of GO into rGO. Therefore the electron ejected from the decomposition of acetaldehyde combines with the holes in rGO and increases the resistance of the ITO–rGO substrate. At higher acetaldehyde concentrations the number of electrons increases, which results in a slight increase in the resistance and baseline sensitivity. Despite this, the sensitivity of the rGO–TM hybrids is significant as the rate in the increment of Rg/Ra values for a rGO–TM hybrid is significantly higher than that in the respective Rg/Ra values exhibited by the corresponding rGO substrate. However, our future plan includes the detailed justification of the p-type semiconducting behavior of rGO.
Usually, gas sensors based on metal oxides such as SnO2 exhibit an n-type semiconducting property, where the resistance of the sensing probe decreases with respect to analyte concentration. In contrast, herein we observed rGO–M hybrids that have p-type semiconducting properties and their ability for sensing acetaldehyde at room temperature.
Conclusions
We have synthesized rGO–M hybrid films on a conductive glass substrate as a sensing probe for acetaldehyde. The films were deposited through a solid phase reduction process and characterized using Raman spectroscopy, powder X-ray diffraction patterns, field emission scanning electron microscopy and electrical conductivity measurements. The PXRD spectra imply the formation of metal oxide/hydroxide on the rGO matrices. The sensitivity for acetaldehyde samples in air was explored within a range of 10–50%. The concentration (of acetaldehyde) dependent increment of resistance indicates an electron–hole recombination process, which is direct evidence of the p-type semiconducting behavior of the hybrids. The rGO–Ni hybrid exhibits the highest sensitivity. The formation of Ni(OOH), a well known p-type semiconductor, resulted in the optimized sensitivity of the rGO–Ni based probe. The linearity of concentration dependent signal variation indicates the possibility for practical applications of all the hybrids except for rGO–Mn. In the near future, we are expecting to devise rGO–metal oxide based p-type semiconductors for the fabrication of inexpensive gas sensors.Acknowledgements
This work was supported by Innovative Areas “Coordination Programming” (area 2107) from the MEXT, Japan.Notes and references
- P. Rai, M. S. Majhi, Y.-T. Yu and J.-H. Lee, RSC Adv., 2015, 5, 76229 RSC.
- D. Chen, H. Feng and J. Li, Chem. Rev., 2012, 112, 6027 CrossRef CAS PubMed.
- F. Li, X. Jiang, J. Zhao and S. Zhang, Nano Energy, 2015, 16, 488 CrossRef CAS.
- M. J. Lu, J. Li, X. Y. Yang, C. A. Zhang, J. Yang, H. Hu and X. B. Wang, Chin. Sci. Bull., 2013, 58, 2698 CrossRef CAS; Y. Ueno, K. Furukawa, K. Hayashi, M. Takamura, H. Hibino and E. Tamechika, Anal. Sci., 2013, 29, 55 CrossRef PubMed; N. S. Green and M. L. Norton, Anal. Chim. Acta, 2015, 853, 127 CrossRef PubMed; S. Liu, L. Zhou, L. Yao, L. Chai, L. Li, G. Zhang, Kankan and K. Shi, J. Alloys Compd., 2014, 612, 126 CrossRef.
- P. O’ Brien, A. Siraki and N. Shangari, Crit. Rev. Toxicol., 2005, 35, 609 CrossRef CAS; S. Stein, Y. Lao, I.-Y. Yang, S. S. Hecht and M. Moriya, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2006, 608, 1 CrossRef PubMed; L. Chen, M. Wang, P. W. Villalta, X. Luo, R. Feuer, J. Jensen, D. K. Hatsukami and S. S. Hecht, Chem. Res. Toxicol., 2007, 20, 108 CrossRef PubMed.
- R. Otson and P. Fellin, Sci. Total Environ., 1988, 77, 95 CrossRef CAS PubMed; A. Vairavamurthy, J. M. Roberts and L. Newman, Atmos. Environ., Part A, 1992, 26, 1965 CrossRef.
- M. Zhou and S. J. Dong, Acc. Chem. Res., 2011, 44, 1232 CrossRef CAS PubMed.
- M. R. Karim, Y. Ikeda, T. Ide, S. Sugimoto, K. Toda, Y. Kitamura, T. Ihara, T. Matsui, T. Taniguchi, M. Koinuma, Y. Matsumoto and S. Hayami, New J. Chem., 2014, 38, 2120 RSC.
- C. Wang, L. Yin, L. Zhang, D. Xiang and R. Gao, Sensors, 2010, 10, 2088 CrossRef CAS PubMed.
- G. Xi, J. Ye, Q. Ma, N. Su, H. Bai and C. Wang, J. Am. Chem. Soc., 2012, 134, 6508 CrossRef CAS PubMed; E. Redel, E. Arsenault, P. G. O’ Brien, N. P. Kherani and G. A. Ozin, Chem. Mater., 2011, 23, 1353 CrossRef; Y. Zhang, Z. Zheng and F. Yang, Ind. Eng. Chem. Res., 2010, 49, 3539 CrossRef; N. G. Cho, H.-S. Woo, J.-H. Lee and I.-D. Kim, Chem. Commun., 2011, 47, 11300 RSC; A. Yu, Z. Liang, J. Cho and F. Caruso, Nano Lett., 2003, 3, 1203 CrossRef.
- N. Yamazoe, Y. Kurokawa and T. Seiyama, Sens. Actuators, B, 1983, 4, 283 CrossRef CAS.
- M. R. Karim, H. Shinoda, M. Nakai, K. Hatakeyama, H. Kamihata, T. Matsui, T. Taniguchi, M. Koinuma, K. Kuroiwa, K. Mohamedally, Y. Matsumoto and S. Hayami, Adv. Funct. Mater., 2013, 23, 323 CrossRef CAS.
- M. R. Karim, K. Hatakeyama, T. Matsui, H. Takehira, T. Taniguchi, M. Koinuma, Y. Matsumoto, T. Akutagawa, T. Nakamura, S.-I. Noro, T. Yamada, H. Kitagawa and S. Hayami, J. Am. Chem. Soc., 2013, 135, 8097 CrossRef CAS PubMed.
- C. Jiang, G. Zhang, Y. Wu, L. Li and K. Shi, CrystEngComm, 2012, 14, 2739 RSC; T. Becker, S. Ahlers, B. Bosch-von, G. Muller and O. Keisewetter, Sens. Actuators, B, 2001, 77, 55 CrossRef CAS.
- L. G. Cançado, K. Takai, T. Enoki, M. Endo, Y. A. Kim, H. Mizusaki, A. Jorio, L. N. Coelho, R. Magalhães-Paniago and M. A. Pimenta, Appl. Phys. Lett., 2006, 88, 163106 CrossRef.
- C. Xu, X. Wang and J. Zhu, J. Phys. Chem. C, 2008, 112, 19841 CAS.
- Y. Ikeda, M. R. Karim, H. Takehira, K. Hatakeyama, T. Matsui, T. Taniguchi, M. Koinuma, Y. Matsumoto and S. Hayami, Chem. Lett., 2014, 43, 486 CrossRef CAS.
- X. H. Xia, J. P. Tu, J. Zhang, X. L. Wang, W. K. Zhang and H. Huang, Sol. Energy Mater. Sol. Cells, 2008, 92, 628 CrossRef CAS.
- A. Gorschinski, G. Khelashvili, D. Schild, W. Habicht, R. Brand, M. Ghafari, H. Bönnemann, E. Dinjusa and S. Behrens, J. Mater. Chem., 2009, 19, 8829 RSC.
- H. I. Hima, X. Xiang, L. Zhang and F. Li, J. Mater. Chem., 2008, 18, 1245 RSC.
- N. Kijima, H. Yasuda, T. Sato and Y. Yoshimura, J. Solid State Chem., 2001, 159, 94 CrossRef CAS.
- C. M. Ghimbwu, J. Schoonman, M. Lumbreras and M. Siadat, Appl. Surf. Sci., 2007, 253, 7483 CrossRef CAS; M. Gautam and H. A. Jayatissa, Solid-State Eletron., 2012, 78, 159 CrossRef.
- J. A. Dirksen, K. Duval and T. A. Ring, Sens. Actuators, B, 2001, 80, 106 CrossRef CAS; C. Zhao, Y. Gu, H. Chen and Z. Jiang, Electrochim. Acta, 2002, 47, 1801 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Raman spectra and FE-SEM images. See DOI: 10.1039/c6qi00058d |
| This journal is © the Partner Organisations 2016 |