Open Access Article
Open Access ArticleNarrow bandgap thienothiadiazole-based conjugated porous polymers: from facile direct arylation polymerization to tunable porosities and optoelectronic properties†
Hassan
Bohra
a,
Si Yu
Tan
b,
Jinjun
Shao
a,
Cangjie
Yang
a,
Amsalu
Efrem
a,
Yanli
Zhao
b and
Mingfeng
Wang
*a
aSchool of Chemical and Biomedical Engineering, Nanyang Technological University, 62 Nanyang Dr., Singapore 637459, Singapore. E-mail: mfwang@ntu.edu.sg
bDivision of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371, Singapore
First published on 29th September 2016
Abstract
Conjugated porous polymers with narrow bandgaps are important for light harvesting in the near infrared region, but are limited by the availability of the appropriate building blocks and synthetic tools. Here we report a series of narrow bandgap conjugated porous polymers synthesized by facile direct arylation polymerization of thiophene-flanked thienothiadiazole with multibrominated monomers with different geometries. The polymer products show strong light absorption in the near infrared region, corresponding to narrow optical bandgaps below 1.3 eV. Under the same polymerization conditions, the morphologies, porosities and optoelectronic properties of the resulting polymers are determined by the chemical structures of the aryl bromides. The synthetic protocol of direct arylation polymerization and the structure–property relationship established in these narrow bandgap conjugated porous polymers will be important for rational material design towards applications such as gas separation/storage and photocatalysis.
Introduction
Conjugated porous polymers (CPPs)1–5 integrated with high porosity, optoelectronic properties and chemical stability are a class of multifunctional organic materials that have attracted much attention owing to their potential applications such as gas storage,6–8 chemosensing,9,10 and photocatalysis.11–13 Similar to linear conjugated polymers, the design and synthesis of CPPs follows a modular approach.14 With careful selection of monomers and polymerization techniques, tunability over a wide range of physical and chemical properties can be achieved.15,16 In particular, bandgap control is crucial in polymers for energy conversion as well as other light-harvesting applications. For instance, incorporation of strong electron-accepting moieties such as benzothiadiazoles and weak electron-donating units such as benzenes and fluorenes in conjugated polymer networks not only reduced the bandgaps, but also improved the photocatalytic activity compared to pure electron-donating networks.17 A similar donor–acceptor (D–A) strategy was used recently by Bonillo et al. to finely tune the bandgap and emission properties of polyphenylene networks by modifying the monomer ratios of different electron-accepting moieties in a Suzuki–Miyaura polymerization protocol.18Traditional synthesis of CPPs involving the aryl C–C bond formation has been carried out by transition-metal catalyzed reactions such as Sonogashira–Hagihara coupling,19 Yamamoto coupling,20 Suzuki–Miyaura coupling,16,21 and Stille coupling.22 Linker-specific polycondensation reactions such as Schiff-base condensation23 and imidization24 reactions have also been vastly utilized in the synthesis of CPPs. Most of these reactions, nevertheless, involve multiple synthetic steps for the functionalization of comonomers. Moreover, some transition-metal catalyzed reactions such as Stille coupling result in the formation of toxic byproducts which are detrimental to the environment.
Direct arylation polymerization (DAP) as an alternative to conventional polymerization reactions for the synthesis of linear π-conjugated polymers has attracted increasing attention in the last decade.25 DAP, which involves the facile one-step coupling of aryl bromides with C–H active arenes without preactivation of C–H bonds using flammable and/or toxic organometallic reagents, has enabled the synthesis of a myriad of linear conjugated polymers with qualities (regioselectivity, molecular weights, etc.) comparable to or even better than those synthesized by conventional coupling techniques.26–29 However, DAP as a synthetic tool for conjugated porous polymers has not been investigated until recently.30 For instance, Liu et al.30a have reported DAP between 1,2,4,5-tetrafluorobenzene and two aryl bromides, 1,3,5-tribomobenzene and tetrakis(4-bromophenyl)methane. Although the resulting polymers showed specific surface areas as high as 1170 m2 g−1, their wide bandgaps limited light absorption in visible and near infrared (NIR) regions. More recently, our group reported a facile one-step DAP using dibromothienophenanzine as a single monomer, resulting in CPPs with a narrow bandgap of 1.5 eV and hierarchical porosity.30b However, the scope of CPPs that could be synthesized using the facile DAP scheme remains rather limited.
Herein, we report the synthesis of a series of narrow-bandgap conjugated porous polymers by DAP of 4,6-di(2-thienyl)thieno[3,4-c][1,2,5]thiadiazole (TTD) with multi-topic aryl bromides. TTD, composed of a strong electron-accepting core of thienothiadiazole flanked by thiophene groups on both sides, is a coplanar π-conjugated narrow bandgap monomer. We speculated that the electron-deficient nature of the thienothiadiazole core could result in effective cleavage of the acidic C–H bonds in the thiophene groups. To understand the reactivity and regioselectivity of TTD, C–H direct arylation coupling between TTD and three monobrominated reagents (MBr-1–3, Scheme 1) was examined, resulting in three donor–acceptor–donor (D–A–D) triads that are easier to purify and characterize than CPPs. Then three representative electron-rich/neutral aryl bromides, 1,3,5-tribromobenzene,15a,17 2,2′,7,7′-tetrabromo-9,9′-spirobifluorene20a,31 and tris(4-bromophenyl)amine32 were used as comonomers in DAP with TTD. The structure–property relationships of TTD-based CPPs were studied through systematic characterization using nuclear magnetic resonance (NMR) spectroscopy, Fourier transform infrared spectroscopy (FTIR), elemental analysis, electron microscopy, porosity analysis and UV-VIS-NIR absorption spectroscopy.
Experimental
General synthesis procedure for TTD-Xs via direct arylation coupling
TTD (80 mg, 0.2614 mmol), MBr-1, -2 or -3 (2.61 mmol), Pd2(dba)3 (0.013 mmol, 11.9 mg), (o-MeOPh)3P (0.02614, 9.2 mg), K2CO3 (1.045 mmol, 144.3 mg) and PivOH (0.137 mmol, 13.3 mg) were added into a reaction vial equipped with a magnetic stirring bar. o-Xylene (1.3 ml, 0.2 M) was added inside a glovebox and the vial was sealed. The reaction was carried out for 24 hours in an oil bath preheated to 120 °C. After completion, the reaction mixture was diluted with 30 mL chloroform and filtered to remove any insoluble matter. Solvent was removed under vacuum and the crude product was separated over silica gel. The pure product was obtained by precipitation in methanol.TTD-1: MBr-1 = 1-bromo-4-hexylbenzene (629.4 mg). Yield: 110.9 mg (95%). 1H NMR (CDCl3, 300 MHz) δ ppm, 7.57 (d, 4H, J = 8.3 Hz), 7.54 (d, 2H, J = 4.1 Hz), 7.30 (d, 2H, J = 4.0 Hz), 7.21 (d, 4H, J = 8.3 Hz), 2.69–2.58 (m, 4H), 1.62 (d, 4H, J = 7.9 Hz), 1.62 (s, 12H), 0.89 (t, 6H, J = 6.9 Hz). EI MS m/z (%) 626 (M+, 93), 605 (10), 568 (8), 499 (13).
TTD-2: MBr-2 = 4-bromotriphenylamine (846.1 mg). Yield: 130.6 mg (80%). 1H NMR (CDCl3, 300 MHz) δ ppm, 7.55–7.52 (m, 4H), 7.50 (d, 2H, J = 2.0 Hz), 7.54 (d, 2H, J = 4.1 Hz), 7.31 (d, 2H, J = 2.0 Hz), 7.29 (d, 4H, J = 1.2 Hz), 7.24 (d, 2H, J = 7.9 Hz), 7.16–7.02 (m, 18H). EI MS m/z (%) 792 (M+, 100), 644 (47), 605 (8), 456 (10).
TTD-3: MBr-3 = 2-bromo-9,9-dioctyl-9H-fluorene (1.22 g). Yield: 121 mg (83%). 1H NMR (CDCl3, 300 MHz) δ ppm, 7.57 (s, 6H, br), 7.61 (s, 4H, br), 7.42 (d, 2H, J = 3.8 Hz), 7.35 (s, 6H, br), 2.07–1.92 (m, 8H), 1.22–0.98 (m, 40H), 0.8 (t, 12H, J = 1 Hz), 0.64 (s, 8H, br). EI MS m/z (%) 1083 (M+, 100), 1060 (8), 897.97 (6), 855 (21).
General synthesis procedure for CPP-Xs via direct arylation polymerization
TTD, PBr-X (C–H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C–Br = 1
C–Br = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Pd2(dba)3 (5 mol%), (o-MeOPh)3P (10 mol%), K2CO3 (400 mol%) and PivOH (50 mol%) were added to a reaction vial charged with a magnetic stirring bar. o-Xylene (0.2 M) was added inside a glove-box and the vial was sealed with a rubber cap. The reaction was carried out at 120 °C in an oil bath for 48 hours. After cooling to room temperature, the reaction mixture was diluted with THF and filtered under vacuum. The solids were washed with methanol, water, dilute HCl and THF and then subjected to Soxhlet extraction with methanol and THF for 24 hours, respectively. The residual solids were collected and dried at 100 °C under vacuum to obtain the final product.
1), Pd2(dba)3 (5 mol%), (o-MeOPh)3P (10 mol%), K2CO3 (400 mol%) and PivOH (50 mol%) were added to a reaction vial charged with a magnetic stirring bar. o-Xylene (0.2 M) was added inside a glove-box and the vial was sealed with a rubber cap. The reaction was carried out at 120 °C in an oil bath for 48 hours. After cooling to room temperature, the reaction mixture was diluted with THF and filtered under vacuum. The solids were washed with methanol, water, dilute HCl and THF and then subjected to Soxhlet extraction with methanol and THF for 24 hours, respectively. The residual solids were collected and dried at 100 °C under vacuum to obtain the final product.
CPP-1: 1,3,5-tribromobenzene (70 mg), TTD (102 mg), Pd2(dba)3 (15.27 mg), (o-MeOPh)3P (11.74 mg), K2CO3 (184.37 mg), PivOH (17.14 mg) and o-xylene (1.67 mL). Yield: 110.9 mg (94%). IR (KBr cm−1): 3059 (br), 1583 (sh), 1555 (sh), 1491 (sh), 1408 (sh), 856 (sh), 833 (sh), 792 (br). Elemental Analysis (%) for C48H18N6S12 Calculated: C, 54.24; H, 1.69; N, 7.91; S, 36.16. Found: C, 51.34; H, 1.88; N, 5.89; S, 26.05.
CPP-2: tris(4-bromophenyl)amine (126 mg), TTD (120 mg), Pd2(dba)3 (17.95 mg), (o-MeOPh)3P (13.816 mg), K2CO3 (216.75 mg), PivOH (20.15 mg) and o-xylene (1.96 mL). Yield: 130.6 mg (71%). IR (KBr cm−1): 3054 (br), 3021 (sh), 1593 (sh), 1529 (sh), 1480 (sh), 1429 (br), 1320 (sh), 1266 (br), 847 (br), 829 (sh), 792 (br). Elemental Analysis (%) for C72H36N8S12 Calculated: C, 61.89; H, 2.58; N, 8.02; S, 27.51. Found: C, 58.65; H, 2.576; N, 6.468; S, 21.09.
CPP-3: 2,2′,7,7′-tetrabromo-9,9′-spirobifluorene (105.4 mg), TTD (102.06 mg), Pd2(dba)3 (15.27 mg), (o-MeOPh)3P (11.74 mg), K2CO3 (184.37 mg), PivOH (17.14 mg) and o-xylene (1.67 mL). Yield: 121 mg (79%). IR (KBr cm−1): 3058 (sh), 1636 (br), 1600 (sh), 1572 (sh), 1527 (br), 1435 (sh), 1410 (sh), 1249 (sh), 1139 (br), 1055 (sh), 854 (sh), 792 (br). Elemental Analysis (%) for C49H20N4S8 Calculated: C, 63.88; H, 2.19; N, 6.08; S, 27.85. Found: C, 57.26; H, 1.98; N, 4.4; S, 19.93.
Results and discussion
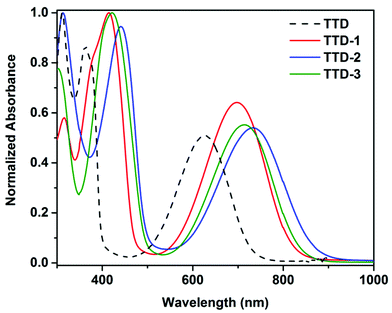
The synthetic routes to the TTD-based small molecular D–A–D triads and CPPs are shown in Scheme 1. These triads serve as model compounds for the respective conjugated polymer networks. A Pd(0) catalyst, Pd2(dba)3, in combination with (o-MeOPh)3P was used in a carboxylate-mediated direct arylation coupling to produce TTD-(1–3).28 The reaction was carried out at 120 °C for 24 hours. All triads were obtained in high yields – 95% for TTD-1, 80% for TTD-2 and 83% for TTD-3. 1H-NMR spectra of the triads (Fig. 1) show the peak from the β-proton of the TTD core at 7.30 ppm in TTD-1, 7.31 ppm in TTD-2 and 7.41 ppm in TTD-3, respectively, suggesting good regioselectivity of TTD under the present reaction conditions. The chemical structures of the triads were further confirmed by mass spectroscopy (Fig. S1†). The relatively high reactivity and regioselectivity of TTD observed in these model reactions are crucial for the following DAP to obtain structurally defined CPPs.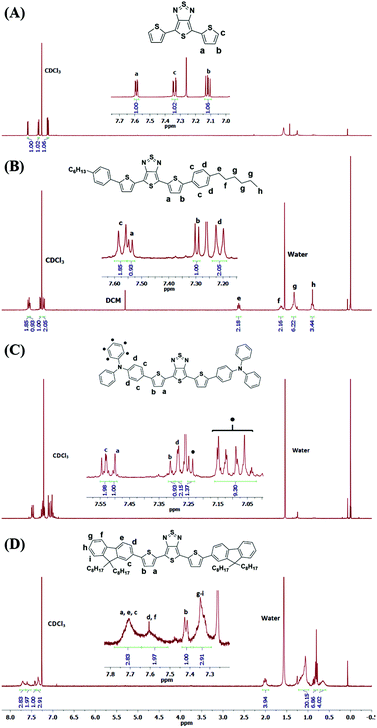 | ||
| Fig. 1 1H-NMR spectra of (A) TTD, (B) TTD-1, (C) TTD-2 and (D) TTD-3 in CDCl3 at 298 K with complete assignment of protons. | ||
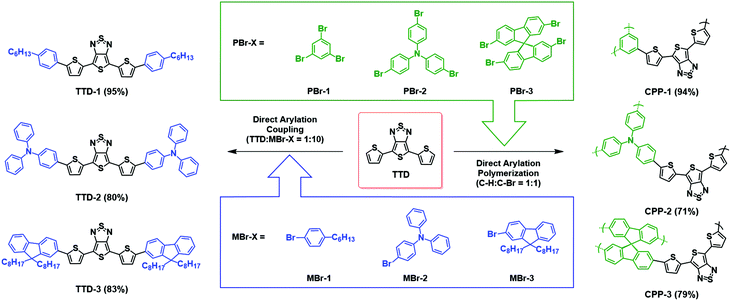
Optical properties of TTD-based triads were studied in chlorobenzene by UV-Vis-NIR absorption spectroscopy (Fig. 2). Absorption maxima of all triads show a significant red shift when compared to the spectrum of TTD, which is mainly attributed to the donor–acceptor interaction between TTD and its donor partner. It can be observed that the extent of the red-shift is proportional to the electron-donating strength of the donor. Among the three D–A–D triads, TTD-2–TTD coupled with two triphenylamine moieties, absorbs at the longest wavelength and has the smallest optical bandgap (Eoptg = 1.38 eV), whereas TTD-1 consisting of phenyl groups has the largest optical bandgap (Eoptg = 1.48 eV). The decrease of the bandgap in the order of TTD-2 < TTD-3 < TTD-1 is consistent with the increasing strength of the electron donating groups (triphenylamine > fluorene > phenyl) in the triads.
TTD-based near infrared conjugated porous polymers (CPP-(1–3)) were synthesized using the same reaction conditions as for the synthesis of TTD-1–3 triads, with the exception of the reaction time and monomer ratio. The DAP28 reaction condition used here was found efficient for the synthesis of benzodithiophene-based linear polymers and the C–H activation of a variety of thiophene-flanked acceptor molecules29a–d including thienothiadiazole29e for the synthesis of donor–acceptor linear polymers. A molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between C–H and C–Br bonds of the monomers was maintained and all the polymerization reactions were carried out for 48 hours to ensure as complete a reaction as possible. Polymers were purified by washing thoroughly with organic (methanol, chloroform and THF) and aqueous solvents (water and diluted HCl) to remove all oligomeric products as well as any byproducts of DAP. The CPPs obtained here appear as green powders that are insoluble in common organic solvents such as THF, chloroform, toluene and chlorobenzene.
1 between C–H and C–Br bonds of the monomers was maintained and all the polymerization reactions were carried out for 48 hours to ensure as complete a reaction as possible. Polymers were purified by washing thoroughly with organic (methanol, chloroform and THF) and aqueous solvents (water and diluted HCl) to remove all oligomeric products as well as any byproducts of DAP. The CPPs obtained here appear as green powders that are insoluble in common organic solvents such as THF, chloroform, toluene and chlorobenzene.
Characterization of the chemical structures of CPP-(1–3) was first carried out using FTIR spectroscopy (Fig. 3). A band at 1489 cm−1 in the FTIR spectra of TTD, which is assigned to the symmetric stretch of the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of thiophene, is clearly present in the spectra of all CPPs. The symmetric C
C bond of thiophene, is clearly present in the spectra of all CPPs. The symmetric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching bands of their respective aryl bromide comonomers appear at 1583 cm−1 in CPP-1, 1480 cm−1 in CPP-2 and 1465 cm−1 in CPP-3, respectively. Stretching vibrations of thiophene rings in TTD were also detected as weak bands in the frequency range between 840 and 860 cm−1. CPP-2 showed the characteristic stretching bands of aromatic C–N bonds at 1320 cm−1 (Fig. 3b). The obvious attenuation of the C–Br stretch band at 1096 cm−1 in CPP-1 suggests the highly efficient direct arylation reaction and the large extent of polymerization. In contrast to CPP-1, small peaks at 1071 and 1060 cm−1 corresponding to the C–Br stretch band were still observed in CPP-2 and CPP-3, respectively, suggesting the lower extent of polymerization in the latter two polymers than the former.
C stretching bands of their respective aryl bromide comonomers appear at 1583 cm−1 in CPP-1, 1480 cm−1 in CPP-2 and 1465 cm−1 in CPP-3, respectively. Stretching vibrations of thiophene rings in TTD were also detected as weak bands in the frequency range between 840 and 860 cm−1. CPP-2 showed the characteristic stretching bands of aromatic C–N bonds at 1320 cm−1 (Fig. 3b). The obvious attenuation of the C–Br stretch band at 1096 cm−1 in CPP-1 suggests the highly efficient direct arylation reaction and the large extent of polymerization. In contrast to CPP-1, small peaks at 1071 and 1060 cm−1 corresponding to the C–Br stretch band were still observed in CPP-2 and CPP-3, respectively, suggesting the lower extent of polymerization in the latter two polymers than the former.
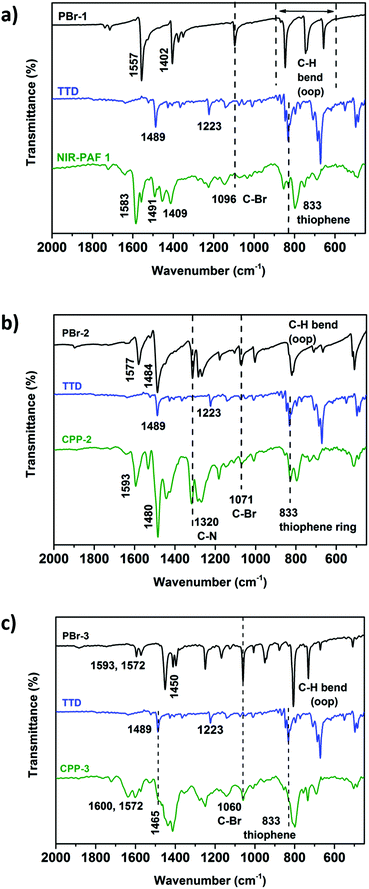 | ||
| Fig. 3 Comparison of FT-IR spectra (fingerprint region) of (a) CPP-1, (b) CPP-2 and (c) CPP-3 shown in green with TTD (blue) and their respective aryl bromide comonomers (black). | ||
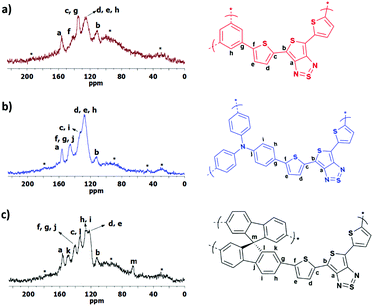
CPP-(1–3) were further characterized by solid state 13C CP/MAS NMR spectroscopy to confirm their structures. 13C NMR spectra of all polymers with complete peak assignments are shown in Fig. 4. A sharp peak around 156 ppm and a broad peak around 112 ppm, which are assigned to the carbons of the thienothiadiazole moiety of TTD, are observed in the spectra of all CPPs. In CPP-1, the broad peaks observed at 141.7 and 135.5 ppm could be attributed to the substituted carbons of the TTD (α-carbon) and the phenyl repeating unit, respectively (Fig. 4a). In addition, the peaks of unsubstituted carbons (C–H) of these monomers overlap into a broad peak located at 125.8 ppm. In CPP-2, the carbon signal from the tertiary amine (C–N) group appears at 145.8 ppm and overlaps with the signals of the substituted carbons of triphenylamine and TTD units (Fig. 4b). Unsubstituted carbons of CPP-2 are assigned to the broad peak at 128 ppm and its shoulder at 132.6 ppm. In CPP-3, the signals from the phenyl rings of spirobifluorene appear as broad peaks between 150 and 124 ppm, as shown in Fig. 4c. The sharp peak at 66.5 ppm is assigned to the quaternary carbon of spirobifluorene. The 13C-NMR results discussed above, which are complementary to the FTIR results, further prove the chemical structures of the target CPP-1–3 obtained under the present direct arylation scheme.
Elemental analysis of CPPs showed some inconsistency between the theoretical (neglecting the contribution of bromide end groups) and experimental values, which is typical of highly conjugated organic networks.30b A better match between the theoretical and experimental mass percentages was observed in CPP-1 compared to CPP-2 and CPP-3. These results are consistent with the FTIR spectra of CPP-2 and CPP-3 (Fig. 3b and c) where the appearance of a weak C–Br band indicates their lower extent of polymerization.
Powder X-ray diffraction (XRD) patterns of all CPPs exhibit broad peaks in the range 2θ = 5–60°, indicating the amorphous nature of these polymers (Fig. S3†). Thermogravimetric analysis shows that all CPPs (Fig. S4†) are stable up to 300 °C under a nitrogen atmosphere, a characteristic of the good thermal stability of such covalent polymer networks.
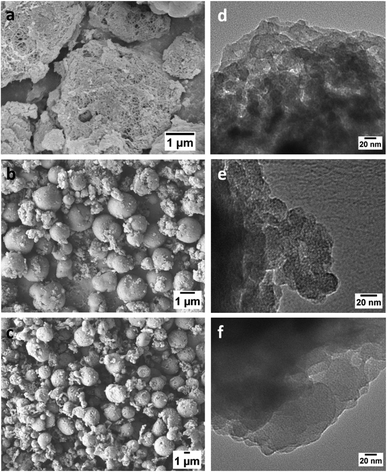
Morphologies of CPP-(1–3) were studied by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). From the SEM image of CPP-1 (Fig. 5), dense networks of thin fibres can be observed most likely due to aggregation of the individual strands. The thickness of the fibres estimated from the SEM images is ∼30 nm (Fig. S8a†).
Both CPP-2 and CPP-3 appear as microspheres with different sizes as seen from their SEM images (Fig. 5b and c). The particles of CPP-3 are approximately twice as large as those of CPP-2. Moreover, the micro-spheres of CPP-2 have a smooth surface, in contrast to the granular and relatively rough surface of CPP-3 (Fig. S5c†). While the high-resolution TEM images (Fig. 5d–f) clearly show the microporous structures of CPP-2 (Fig. 5e) and CPP-3 (Fig. 5f), the microporous nature is not obvious in CPP-1 (Fig. 5d), presumably due to its smaller pore sizes and tighter π–π stacking caused by the relatively small and flat phenyl repeating unit in CPP-1.
Porosities of CPP-1, CPP-2 and CPP-3 were studied in physisorption experiments using nitrogen as the sorbate molecule. Sorption isotherms of CPPs measured at 77 K are shown in Fig. 6. All polymers show a combination of Type II and Type IV sorption isotherms according to IUPAC classification. The uptake of N2 at relatively low pressures p/po < 0.1 is much lower compared to previously reported porous organic polymers,1,6,14 suggesting the relatively low microporosity in these polymers. In contrast, a sharp increase of N2-uptake was observed at p/po = 0.9–1, an indication of the presence of dominating meso- and macropores from the inter-particular voids in these polymers. A small degree of hysteresis was observed in the isotherms of CPP-1 in the relatively high pressure range (0.5 < p/po < 0.99). This can be explained by the presence of pores with narrow openings that restrict the access of nitrogen molecules. Alternatively, this could also be attributed to the swelling of the polymer framework due to the dissolution of the adsorbate into the non-porous sections of polymer during the adsorption cycle.33
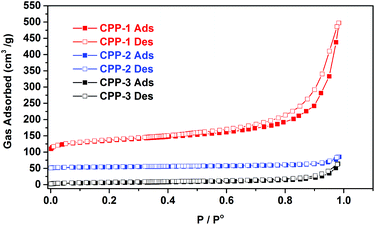 | ||
| Fig. 6 N2 adsorption–desorption isotherms of CPP-(1–3) measured at 77 K. For clarity, the isotherms of CPP-1 and CPP-2 were shifted vertically by 50 cm3 g−1, respectively. | ||
Key porosity properties of CPPs are listed in Table 1. Among the three polymers, CPP-1 with fibrillar networks shows the largest Brunauer–Emmett–Teller (BET) surface area of 128.3 m2 g−1, which is significantly higher than those of the CPP-2 (14.2 m2 g−1) and CPP-3 (20.63 m2 g−1) microspheres. Pore size distribution (PSD) profiles of CPPs were calculated using the NL-DFT method (Fig. S7†). All CPPs have a broad PSD ranging from 14 Å to well beyond 200 Å. The structure–porosity relationship that we observed here in CPP-1–3 is similar to that in previously reported conjugated microporous polymers (CMPs).15,19,34 For instance, the lower surface area of CPP-2 as compared to CPP-1, which is consistent with the morphologies of these polymers as shown in the SEM images (Fig. 5), may be attributed to the larger size of the triphenylamine moiety. Cooper and co-workers also reported that increasing the size of monomers in an A2 + B3 synthetic model led to polymers with lower surface areas.15a
| Species |
S
BET![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (m2 g−1) a (m2 g−1) |
S
DFT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (m2 g−1) b (m2 g−1) |
V
micro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (cm3 g−1) c (cm3 g−1) |
V tot, DFT (cm3 g−1) |
|---|---|---|---|---|
| a Specific surface area calculated from the adsorption branch of the N2 isotherm using the BET method. b Specific surface area calculated using the NL-DFT method for slit pores with the equilibrium model. c Micro-pore volume calculated from the adsorption branch of the N2 isotherm using the t-plot method. | ||||
| CPP-1 | 128.3 | 95.36 | 0 | 0.52 |
| CPP-2 | 14.2 | 10.9 | 0 | 0.04 |
| CPP-3 | 20.63 | 17.2 | 0 | 0.08 |
Optical properties of CPPs in chlorobenzene dispersions were studied by UV-Vis-NIR spectroscopy (Fig. 7). Compared to the absorption spectra of the D–A–D triads, all the three polymers (CPP-(1–3)) show a broad absorption across the visible and the near infrared regions. Despite some light scattering effect of the polymer dispersions which made it difficult to estimate the wavelength of the onset absorption, the band-edge absorption peaks of CPP-1–3 at 772, 840 and 727 nm, respectively, remain well-resolved. The optical bandgaps of CPP-1–3 follow the trend of CPP-2 < CPP-1 ≈ CPP-3 (Table S1†). CPP-2 absorbs at the longest wavelength in the NIR region and has the smallest bandgap, largely attributed to the strong donor–acceptor interactions between triphenylamine and TTD. The slightly larger bandgap of CPP-3 vs. CPP-1 could be attributed to its lowest extent of polymerization as reflected from the FTIR spectrum (Fig. 3 and S2†).
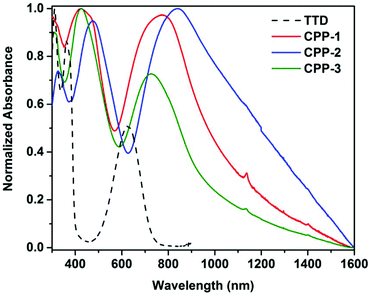 | ||
| Fig. 7 UV-Vis-NIR absorption spectra of CPP-(1–3) in chlorobenzene dispersions. UV-Vis spectrum of TTD is plotted for comparison. | ||
Electrochemical properties of CPPs vs. TTD-based triads were evaluated by cyclic voltammetry (CV). While all triads showed reversible oxidation and quasi-reversible reduction peaks (Fig. S8†), irreversible oxidation and reduction peaks were observed in all the three CPPs (Fig. 8). Oxidation and reduction potentials of CPPs provide further insight into their electronic structures. Oxidation potential (Eox) of CPP-3 at 0.74 V was the highest among the three CPPs, followed by CPP-1 (Eonsetox = 0.68 eV) and CPP-2 (Eonsetox = 0.65 eV). The HOMO levels of CPP-1–3 estimated from the CV results are −5.48, −5.45 and −5.54 eV, respectively. A similar trend was observed for the reduction potential as well, resulting in the LUMO levels which are −4.07, −4.11 and −4.03 eV for CPP-1–3, respectively. These results imply that the extended conjugation of TTD with an electron-donor led to a cathodic shift of the oxidation potentials and therefore an increase of their HOMO energy levels. The extent of the shift is proportional to the strength of the donor. As a consequence, the push–pull effect between TTD and donor moieties results in a systematic narrowing of the HOMO–LUMO gaps of CPPs such that the electrochemical bandgaps follow the order of CPP-2 < CPP-1 < CPP-3 (Table 2). The larger bandgap and the lower HOMO energy level of CPP-3 compared to CPP-1 can be explained by the lower degree of polymerization in the former. These results are consistent with the optical bandgaps of these CPP polymers.
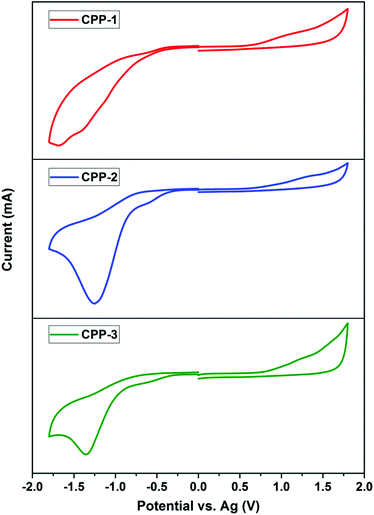 | ||
| Fig. 8 Cyclic voltammograms of CPP-(1–3) in 0.1 M solution of Bu4NPF6 in dry CH3CN as the supporting electrolyte. | ||
| Species | E onsetox (V) | E onsetred (V) |
E
HOMO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (eV) a (eV) |
E
LUMO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (eV) b (eV) |
E Elecg (eV) | λ edge (nm) | E optg (eV) |
|---|---|---|---|---|---|---|---|
| a E HOMO = −(Eonsetox + 4.8). b E LUMO = −(Eonsetred + 4.8). c Optical bandgaps of CPP-1 and CPP-3 were determined by a rough estimation of the onset wavelengths. Onset of CPP-2 is too steep for determination of an onset wavelength. | |||||||
| TTD-1 | 0.739 | −0.694 | −5.54 | −4.11 | 1.43 | 838 | 1.48 |
| TTD-2 | 0.592 | −0.701 | −5.39 | −4.09 | 1.30 | 898 | 1.38 |
| TTD-3 | 0.724 | −0.671 | −5.52 | −4.13 | 1.39 | 855 | 1.45 |
| CPP-1 | 0.681 | −0.726 | −5.48 | −4.07 | 1.41 | 1055c | 1.17c |
| CPP-2 | 0.652 | −0.695 | −5.45 | −4.11 | 1.34 | — | — |
| CPP-3 | 0.739 | −0.774 | −5.54 | −4.03 | 1.51 | 943c | 1.31c |
While TTD-based linear narrow-bandgap polymers have shown potential applications for organic NIR photodetectors and field effect transistors,35 we expect that the 2D/3D TTD-conjugated porous networks described above would find applications such as photocatalysis to harvest light in the NIR region. Moreover, incorporation of strong acceptor molecules in polymer networks of π-conjugated porous polymers has been demonstrated as an efficient strategy for improving their performance as heterogeneous photocatalysts.17,36 TTD-based polymers with weak electron donors may prevent the fast recombination of excitons favouring the formation of a triplet state. Studies to investigate the potential of these TTD-based CPPs as heterogeneous photocatalysts are underway and will be the subject of a future publication.
Conclusions
In summary, three narrow bandgap conjugated porous polymers (CPPs) have been synthesized by direct arylation polymerization of thiophene-flanked thienothiadiazole (TTD) – a low bandgap electron-accepting building block – with polytropic aryl bromides of various geometries. Direct arylation coupling between TTD and a series of mono-brominated aryls resulted in small molecular D–A–D triads in high yields, suggesting the relatively high reactivity and regioselectivity of TTD. Under the same polymerization conditions, the morphologies and porosities of the CPPs were determined by the structure of the aryl monomers. Specifically, DAP coupling between TTD and 1,3,5-tribomobenzene resulted in fibrillar networks that showed significantly higher porosity than the other two CPPs with 2,2′,7,7′-tetrabromo-9,9′-spirobifluorene and tris(4-bromophenyl)amine as comonomers, respectively. The bandgaps of the CPPs were determined by the D–A intramolecular charge transfer. Among the three CPPs, CPP-2 composed of TTD and triphenylamine as repeating units showed the narrowest bandgap below 1.3 eV due to the largest extent of D–A charge transfer. These experimental results demonstrate that a rational selection of monomers enables the synthesis of a series of narrow bandgap conjugated porous polymers with tunable porosities, morphologies and optoelectronic properties.Acknowledgements
M. W. is grateful for the funding support by a start-up grant (M4080992.120) of the Nanyang Assistant Professorship from the Nanyang Technological University, and AcRF Tier 1 (M4011061.120, RG49/12) from the Ministry of Education – Singapore. Mass spectroscopy was carried out in the Proteomics and Mass Spectroscopy Services facility, School of Biological Sciences, Nanyang Technological University. H. B. gratefully acknowledges the Ph.D. research scholarships from Nanyang Technological University.Notes and references
- R. Dawson, A. Cooper and D. Adams, Prog. Polym. Sci., 2012, 37, 530–563 CrossRef CAS.
- Q. Liu, Z. Tang, M. Wu and Z. Zhou, Polym. Int., 2013, 63, 381–392 CrossRef.
- N. McKeown and P. Budd, Macromolecules, 2010, 43, 5163–5176 CrossRef CAS.
- A. Slater and A. Cooper, Science, 2015, 348, 8075 CrossRef PubMed.
- A. Thomas, Angew. Chem., Int. Ed., 2010, 49, 8328–8344 CrossRef CAS PubMed.
- Z. Xiang and D. Cao, J. Mater. Chem. A, 2013, 1, 2691–2718 CAS.
- R. Dawson, A. Cooper and D. Adams, Polym. Int., 2013, 62, 345–352 CrossRef CAS.
- S. Hug, L. Stegbauer, H. Oh, M. Hirscher and B. Lotsch, Chem. Mater., 2015, 27, 8001–8010 CrossRef CAS.
- (a) H. Ma, F. Li, P. Li, H. Wang, M. Zhang, G. Zhang, M. Baumgarten and K. Müllen, Adv. Funct. Mater., 2016, 26, 2025–2031 CrossRef CAS; (b) S. Bandyopadhyay, P. Pallavi, A. Anil and A. Patra, Polym. Chem., 2015, 6, 3775–3780 RSC.
- (a) C. Gu, N. Huang, J. Gao, F. Xu, Y. Xu and D. Jiang, Angew. Chem., Int. Ed., 2014, 53, 4850–4855 CrossRef CAS PubMed; (b) L. Xiang, Y. Zhu, S. Gu, D. Chen, X. Fu, Y. Zhang, G. Yu, C. Pan and Y. Hu, Macromol. Rapid Commun., 2015, 36, 1566–1571 CrossRef CAS PubMed.
- Z. Wang, S. Ghasimi, K. Landfester and K. Zhang, Adv. Mater., 2015, 27, 6265–6270 CrossRef CAS PubMed.
- C. Gu, N. Huang, Y. Chen, L. Qin, H. Xu, S. Zhang, F. Li, Y. Ma and D. Jiang, Angew. Chem., Int. Ed., 2015, 54, 13594–13598 CrossRef CAS PubMed.
- J. Luo, X. Zhang and J. Zhang, ACS Catal., 2015, 5, 2250–2254 CrossRef CAS.
- Y. Xu, S. Jin, H. Xu, A. Nagai and D. Jiang, Chem. Soc. Rev., 2013, 42, 8012–8031 RSC.
- (a) J. Jiang, F. Su, A. Trewin, C. Wood, H. Niu, J. Jones, Y. Khimyak and A. Cooper, J. Am. Chem. Soc., 2008, 130, 7710–7720 CrossRef CAS PubMed; (b) R. Dawson, A. Laybourn, Y. Khimyak, D. Adams and A. Cooper, Macromolecules, 2010, 43, 8524–8530 CrossRef CAS.
- (a) Y. Xu, A. Nagai and D. Jiang, Chem. Commun., 2013, 49, 1591–1593 RSC; (b) Z. Xie, S. Yoon and S. Park, Adv. Funct. Mater., 2010, 20, 1638–1644 CrossRef CAS.
- K. Zhang, Z. Vobecka, K. Tauer, M. Antonietti and F. Vilela, Chem. Commun., 2013, 49, 11158 RSC.
- B. Bonillo, R. Sprick and A. Cooper, Chem. Mater., 2016, 28, 3469–3480 CrossRef CAS.
- (a) J. Jiang, F. Su, A. Trewin, C. Wood, N. Campbell, H. Niu, C. Dickinson, A. Ganin, M. Rosseinsky, Y. Khimyak and A. Cooper, Angew. Chem., Int. Ed., 2007, 46, 8574–8578 CrossRef CAS PubMed; (b) E. Stöckel, X. Wu, A. Trewin, C. Wood, R. Clowes, N. Campbell, J. Jones, Y. Khimyak, D. Adams and A. Cooper, Chem. Commun., 2009, 212–214 RSC.
- (a) J. Schmidt, M. Werner and A. Thomas, Macromolecules, 2009, 42, 4426–4429 CrossRef CAS; (b) X. Yang, S. Yao, M. Yu and J. Jiang, Macromol. Rapid Commun., 2014, 35, 834–839 CrossRef CAS PubMed.
- G. Cheng, T. Hasell, A. Trewin, D. Adams and A. Cooper, Angew. Chem., Int. Ed., 2012, 51, 12727–12731 CrossRef CAS PubMed.
- P. Li, T. Schon and D. Seferos, Angew. Chem., Int. Ed., 2015, 54, 9361–9366 CrossRef CAS PubMed.
- Y. Zhu, H. Long and W. Zhang, Chem. Mater., 2013, 25, 1630–1163 CrossRef CAS.
- (a) J. Weber, Q. Su, M. Antonietti and A. Thomas, Macromol. Rapid Commun., 2007, 28, 1871–1876 CrossRef CAS; (b) M. Liebl and J. Senker, Chem. Mater., 2013, 25, 970–980 CrossRef CAS.
- (a) A. Facchetti, L. Vaccaro and A. Marrocchi, Angew. Chem., Int. Ed., 2012, 51, 3520–3523 CrossRef CAS PubMed; (b) K. Wang and M. F. Wang, Curr. Org. Chem., 2013, 17, 999–1012 CrossRef CAS; (c) L. Mercier and M. Leclerc, Acc. Chem. Res., 2013, 46, 1597–1605 CrossRef CAS PubMed; (d) K. Okamoto, J. Zhang, J. Housekeeper, S. Marder and C. Luscombe, Macromolecules, 2013, 46, 8059–8078 CrossRef CAS; (e) S. Kowalski, S. Allard, K. Zilberberg, T. Riedl and U. Scherf, Prog. Polym. Sci., 2013, 38, 1805–1814 CrossRef CAS; (f) A. Rudenko and B. Thompson, J. Polym. Sci., Part A: Polym. Chem., 2014, 53, 135–147 CrossRef; (g) P. Morin, T. Bura and M. Leclerc, Mater. Horiz., 2016, 3, 11–20 RSC.
- (a) M. Sévignon, J. Papillon, E. Schulz and M. Lemaire, Tetrahedron Lett., 1999, 40, 5873–5876 CrossRef; (b) J. Hassan, E. Schulz, C. Gozzi and M. Lemaire, J. Mol. Catal. A: Chem., 2003, 195, 125–131 CrossRef CAS; (c) Q. Wang, R. Takita, Y. Kikuzaki and F. Ozawa, J. Am. Chem. Soc., 2010, 132, 11420–11421 CrossRef CAS PubMed; (d) W. Lu, J. Kuwabara and T. Kanbara, Macromolecules, 2011, 44, 1252–1255 CrossRef CAS; (e) P. Berrouard, A. Najari, A. Pron, D. Gendron, P. Morin, J. Pouliot, J. Veilleux and M. Leclerc, Angew. Chem., Int. Ed., 2011, 51, 2068–2071 CrossRef PubMed.
- (a) R. Matsidik, H. Komber, A. Luzio, M. Caironi and M. Sommer, J. Am. Chem. Soc., 2015, 137, 6705–6711 CrossRef CAS PubMed; (b) J. Pouliot, B. Sun, M. Leduc, A. Najari, Y. Li and M. Leclerc, Polym. Chem., 2015, 6, 278–282 RSC; (c) P. Morin, T. Bura, B. Sun, S. Gorelsky, Y. Li and M. Leclerc, ACS Macro Lett., 2015, 4, 21–24 CrossRef CAS.
- X. Wang and M. Wang, Polym. Chem., 2014, 5, 5784–5792 RSC.
- (a) K. Wang, G. Wang and M. Wang, Macromol. Rapid Commun., 2015, 36, 2162–2170 CrossRef CAS PubMed; (b) X. Wang, K. Wang and M. Wang, Polym. Chem., 2015, 6, 1846–1855 RSC; (c) H. Bohra, J. Shao, S. Huang and M. Wang, Tetrahedron Lett., 2016, 57, 1497–1501 CrossRef CAS; (d) J. Huang, K. Wang, S. Gupta, G. Wang, C. Yang, S. Mushrif and M. Wang, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 2015–2031 CrossRef CAS; (e) J. Shao, G. Wang, K. Wang, C. Yang and M. Wang, Polym. Chem., 2015, 6, 6836–6844 RSC.
- (a) D. Liu, Q. Chen, Y. Zhao, L. Zhang, A. Qi and B. Han, ACS Macro Lett., 2013, 2, 522–526 CrossRef CAS; (b) A. Efrem, K. Wang, P. Amaniampong, C. Yang, S. Gupta, H. Bohra, S. Mushrif and M. Wang, Polym. Chem., 2016, 7, 4862–4866 RSC.
- Q. Chen, J. Wang, F. Yang, D. Zhou, N. Bian, X. Zhang, C. Yan and B. Han, J. Mater. Chem., 2011, 21, 13554–13560 RSC.
- (a) C. Hua, A. Rawal, T. Faust, P. Southon, R. Babarao, J. Hook and D. D'Alessandro, J. Mater. Chem. A, 2014, 2, 12466 RSC; (b) D. Wang, L. Li, W. Yang, Y. Zuo, S. Feng and H. Liu, RSC Adv., 2014, 4, 59877–59884 RSC.
- J. Weber, J. Schmidt, A. Thomas and W. Böhlmann, Langmuir, 2010, 26, 15650–15656 CrossRef CAS PubMed.
- (a) S. Ren, R. Dawson, D. Adams and A. Cooper, Polym. Chem., 2013, 4, 5585–5590 RSC; (b) B. Kim, N. Park, S. Lee, H. Kim and S. Son, Polym. Chem., 2015, 6, 7363–7367 RSC.
- (a) X. Gong, M. Tong, Y. Xia, W. Cai, J. Moon, Y. Cao, G. Yu, C. Shieh, B. Nilsson and A. Heeger, Science, 2009, 325, 1665–1667 CrossRef CAS PubMed; (b) J. Yuen, R. Kumar, J. Seifter, S. Valouch, D. Zakhidov, D. Moses, U. Lemmer, A. Heeger and F. Wudl, J. Am. Chem. Soc., 2011, 133, 19602–19605 CrossRef CAS PubMed.
- (a) Z. Wang, S. Ghasimi, K. Landfester and K. Zhang, Chem. Commun., 2014, 50, 8177 RSC; (b) K. Zhang, D. Kopetzki, P. Seeberger, M. Antonietti and F. Vilela, Angew. Chem., Int. Ed., 2013, 52, 1432–1436 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Mass spectra and cyclic voltammograms of TTD-(1–3). TGA, PXRD patterns, BET specific surface area plots and NL-DFT pore size distribution of CPP-(1–3). See DOI: 10.1039/c6py01453d |
| This journal is © The Royal Society of Chemistry 2016 |