A novel naphthalimide scaffold based iodonium salt as a one-component photoacid/photoinitiator for cationic and radical polymerization under LED exposure
Abstract
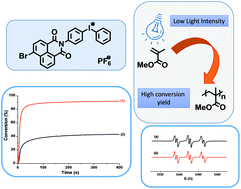
A strong drawback of the photoinitiators of cationic polymerization or photoacids is the photosensitivity for short and energetic wavelengths preventing their general use (specialized photochemical equipment with safety concerns must be used). In the present paper, a novel iodonium salt bearing a naphthalimide moiety (naphthalimide-Ph-I+-Ph) is proposed as a one-component photoinitiator/photoacid operating at longer and safer wavelengths (i.e. violet light emitting diodes at 365, 385 nm and 395 nm). It allows the polymerization of various formulations (methacrylates, epoxides, vinyl ethers). A high reactive function conversion for multifunctional monomers can be achieved: e.g. 50% for a diepoxide under air, >90% for a divinylether (with a very high rate of polymerization Rp), almost 100% for an epoxide/vinyl ether blend (very high Rp) under air, and 85% for methacrylates (high Rp) in laminate (43% under air). These results are above the ones obtained with a thianthrenium salt chosen as a reference e.g. a lower epoxy conversion ∼25% and a clearly lower Rp for the diepoxide polymerization. ESR-spin trapping, laser flash photolysis, steady state photolysis and molecular orbitals calculations support the formation of Ph˙ and naphthalimide-Ph-I˙+ as well as the generation of H+, thereby explaining the photoinitiation step mechanism.

 Please wait while we load your content...
Please wait while we load your content...