Open Access Article
Open Access ArticleChromatography-free synthesis of monodisperse oligo(ethylene glycol) mono-p-toluenesulfonates and quantitative analysis of oligomer purity†
Adam M.
Wawro
a,
Takahiro
Muraoka
abc and
Kazushi
Kinbara
*ab
aInstitute of Multidisciplinary Research for Advanced Materials, Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai 980-8577, Japan. E-mail: kkinbara@bio.titech.ac.jp
bGraduate School of Bioscience and Biotechnology, Tokyo Institute of Technology, 4259 Nagatsuta-cho, Midori-ku, Yokohama 226-8501, Japan
cPRESTO, Japan Science and Technology Agency, 4-1-8 Honcho, Kawaguchi, Saitama 332-0012, Japan
First published on 29th February 2016
Abstract
Poly(ethylene glycol) (PEG) is a common building block for complex functional molecules. Because of its chain flexibility and hydrophilicity, it is widely used in bioconjugation chemistry. Modern applications require PEGs of high purity, hence well-defined, monodisperse PEG oligomers are gaining more attention. Here we report a large-scale synthetic procedure for monodisperse oligo(ethylene glycol) mono-p-toluenesulfonates, convenient intermediates of various heterobifunctional PEG derivatives. This method features no chromatographic purification, low total material cost and high purity of the products. In addition, oligomer dispersity of the products is determined quantitatively using reverse-phase HPLC and a number of bioconjugation-related functionalized PEG derivatives are prepared from the synthesized toluenesulfonates.
Introduction
Poly(ethylene glycol) (PEG)‡ derivatives have found numerous applications in modern chemistry, especially as building blocks for dendrimers,1–3 thermoresponsive materials,4,5 hydrogels,6 self-assembling systems7,8 and surface modifying agents.9,10 PEG-based compounds are also widely used in protein chemistry for crosslinking,11 conjugation with small molecules12,13 and PEGylation.14–18 Particularly, heterobifunctional PEG linkers are promising components of rapidly developing antibody–drug conjugates.In the recent two decades, monodisperse PEGs (i.e. single chemical compounds of well-defined length) have received significant attention as potential next-generation cross-linking reagents.19 The advantages of using monodisperse PEGs include uniform behaviour of all molecules at the molecular level, well-defined dimensions, simple interpretation of MS spectra, ability of being analysed by methods restricted to discrete molecular structures, e.g. XRD,5 and high batch-to-batch reproducibility, a feature highly important in the pharmaceutical industry. For instance, monodispersity of the PEG chain in a PEGylated drug is preferable during the drug registration process.
Although monodispersity offers significant benefits, synthesis of well-defined PEG oligomers requires remarkably more time and resources than simple polymerization. Various synthetic approaches concerning the synthesis of monodisperse PEGs have been proposed in the last two decades. Initial studies focused on synthesis of simple, symmetric PEGs.20–26 However, unmodified PEG chains have limited applications and preferably asymmetric derivatives are of more practical interest.27–29 Even though it is relatively easy to prepare a symmetric derivative from a corresponding unsubstituted PEG, efficient and selective monosubstitution, leading to an asymmetrically modified molecule, is challenging, particularly in the case of long oligomers.30–32 In order to solve this issue, synthesis of asymmetric monodisperse PEGs has been tackled in a number of papers, especially in the last two years.31,33–38 New concepts designed to reduce experimental complexity included customised chemical structures and tags facilitating chromatographic purification34,36,37 and the development of new pro-asymmetric building blocks.38,39 However, although significant progress has been made, all of these processes still suffer from multiple chromatographic purifications and relatively low overall yields, compared to the syntheses of the corresponding symmetric PEGs. Due to this, asymmetric monodisperse PEGs are still not as accessible as their polydisperse counterparts.
Results and discussion
Motivated by the need for large quantities of pure, monodisperse PEG heterobifunctional derivatives, we developed a synthetic method featuring both oligomer purity and synthetic simplicity. The key compounds of our approach are oligo(ethylene glycol) mono-p-toluenesulfonates (PEGn-Ts). This class of compounds was prepared in a chromatography-free, simple process in high yield and in high purity. As the two end groups show completely different reactivities, PEGn-Ts can be further modified to yield various heterobifunctional PEG-based compounds, suitable for diverse applications. Moreover, we determined oligomer purity of the obtained PEGn-Ts using RP-HPLC and compared the results with previously discussed mass-spectrometric procedures.33,38,40 We found that MS-based methods tend to overestimate the actual purity of the analyte, thus are not suitable for precise dispersity analysis.Conceptually, we employed the unidirectional iterative coupling mode for chain elongation.33 Although being inferior to other modes in terms of chain length growth per coupling reaction, this approach allows limiting the number of unique reaction types and retaining initially introduced chain end-groups’ asymmetry.
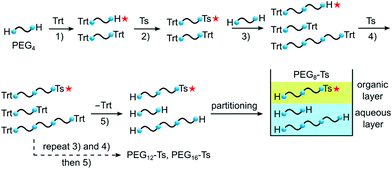
Because mono- and disubstituted PEG derivatives are difficult to separate without the chromatographic process, chromatography-free monodisperse PEG synthetic methods are challenging, especially if the product purity is a key factor. The highlight of our approach is how the side products are managed (Scheme 1). In our method all generated PEG-type by-products are consciously transferred to the following steps and after the final reaction are easily removed by liquid–liquid extraction. This was possible due to the unique behaviour of PEG derivatives: unsubstituted, free PEG chains show strong affinity to the aqueous phase, while p-toluenesulfonyl esters of even relatively long PEG chains (n = 16) partition exclusively into the organic layer. Thus, if all the undesired by-products are eventually transformed into free PEG chains, simple extraction can efficiently separate them from the product. To achieve this goal, two orthogonally cleavable end groups were used. Conventional triphenylmethyl (trityl, Trt) was used as the main protecting group, present from the first to the last step, acting as both a hydroxy protecting group and a hydrophobic tag. The other one, the p-toluenesulfonyl group (Ts), had three functions: a leaving group for Williamson ether synthesis, a hydrophobic tag facilitating chromatography-free purification and the derivatisable functional group expanding the utility of the final asymmetric product. As the PEG building block, we used tetra(ethylene glycol) (PEG4), the longest PEG oligomer available in high purity at a low price.
Initially, PEG4 was treated with trityl chloride to yield Trt-PEG4 (1) contaminated with Trt-PEG4-Trt, as confirmed by 1H NMR and mass spectrometry (Scheme 2). This reaction proceeded swiftly at room temperature and the excess of pyridine was removed by co-evaporation with toluene. In some previous methods,25,26 at this step the presence of the symmetric by-product Trt-PEG4-Trt was neglected, because it was assumed that vast excess of PEG4 was used. However, this contamination would eventually affect oligomer purity of the final compound in the chromatography-free processes.
In the following step Trt-PEG4 was tosylated,41 giving asymmetric Trt-PEG4-Ts (2). The reaction proceeded quickly at 0 °C, and then the remaining tosyl chloride was hydrolysed at room temperature. As long as the mixture is vigorously stirred and the mass transfer between THF-rich and aqueous phases is efficient, PEG tosylates are not hydrolysed under these conditions. It should be also noted that the ditrityl by-product, Trt-PEG4-Trt, transferred from the first step, remains inert at this and the following steps, until all trityl groups are removed.
Successively, tosylate 2 was coupled via Williamson ether synthesis with excess of PEG4. Importantly, apart from the desired Trt-PEG8 (3), a product of double coupling, namely Trt-PEG12-Trt, appeared in a small quantity as well. However, similarly to the previously discussed Trt-PEG4-Trt, it remains inert during the following reactions. The ether synthesis step is responsible for the generation of undesired PEG(n−1) derivatives, generated by base-induced depolymerisation of PEG.42 Among several previously employed conditions for the Williamson coupling, we found that using the NaH/THF system26 at 40 °C provided a good balance between the reaction progress and the depolymerisation rate.
The tosylation/coupling iterative process was repeated until the desired chain length was obtained. Then, the tosylation was performed once more, followed by catalytic hydrogenation to cleave all trityl groups. The produced triphenylmethane was removed roughly by precipitation and washed out completely with hexane. During the reaction, the symmetric, undesired ditrityl PEG derivatives had been hydrogenated into the corresponding symmetric unsubstituted PEG chains. Then, the desired PEGn-Ts was separated from free PEGs by liquid–liquid extraction with ethyl acetate and brine.
Although during the final extraction the accumulated by-products can be easily removed, our procedure does not solve the common problem of PEG(n−1)-Ts and PEG(n+1)-Ts impurities. The one-unit-shorter oligomers appear during Williamson coupling as products of base-induced depolymerisation and both PEG(n−1)-Ts and PEG(n+1)-Ts are generated from PEG3 and PEG5, respectively, present in commercially available PEG4 in small quantities. Thus, the quality of the final product is dependent both on the quality of the purchased PEG4 and generation of the shorter oligomers during the Williamson coupling.
Following this methodology, we successfully prepared PEG8-Ts (5) in the 50 g scale and confirmed that this method can be further extended to the longer oligomers, PEG12-Ts (8) and PEG16-Ts (11), by simply repeating the coupling/tosylation steps. The number of steps required for the whole procedure is expressed as 2n + 3, where n is a number of chain extensions. Therefore, PEG8-Ts was prepared in 5 steps (overall yield 72%), PEG12-Ts in 7 steps (overall yield 63%) and PEG16-Ts in 9 steps (overall yield 62%).§
For successful purification, the desired monotosylate should be partitioned into the organic layer during extraction. As the chain becomes longer, the PEGn-Ts molecule loses its hydrophobic character and isolation by extraction becomes more difficult. We investigated this issue and found that oligomers longer than PEG24-Ts do not have sufficiently high affinity to the organic layer and are lost during extraction. Therefore, our method cannot be applied for the synthesis of derivatives longer than PEG24-Ts (corresponding roughly to conventional polydisperse PEG1000).
As our method requires no chromatographic purification, a process seriously affecting the total cost and limiting the reaction scale, this procedure can be further scaled-up, even to an industrial scale. With chromatography-free design and optimized amounts of solvents and reactants we decreased the total synthetic cost significantly, compared to the established processes. Because the volume of the required solvents was reduced to a minimum, the overall volume of the liquids during the workup is low, allowing using standard-size laboratory glassware even for large-scale syntheses. For instance, synthesis and workup of ca. 100 g of intermediate 4 were done using a 1 L round-bottom flask and separation funnel. In addition to the chromatography-free design, our synthetic methodology provides significantly higher overall yields than the established methods and requires neither metal catalysts nor elaborate reagents.33,34,36,38
With large quantities of PEGn-Ts oligomers in hand, we were determined to measure the product oligomer purity as precisely as possible. Recent findings suggested that MALDI-TOF MS is superior to ESI-TOF MS in this matter.40 However, mass-spectrometry-based methods are intrinsically not quantitative. Even though MALDI-TOF MS had been shown to give reasonable approximations at a certain laser power and ionic additive concentration,43 we tackled the purity determination problem from a different angle. With the p-toluenesulfonyl group acting as a chromophore for UV detection, we employed reverse-phase (RP) HPLC to determine PEGn-Ts monodispersity. This method was expected to be suitable for quantitative analysis of a mixture of molecules with the same chromophore.
The separation of single oligomers by HPLC was optimized using a mixture of monotosylates prepared directly from polydisperse PEG400, consisting of various oligomers (Fig. 1a, b and S2g, h†). The desired separation quality, i.e. complete resolution of all present oligomers, was obtained with a conventional reverse-phase octadecyl-modified silica column at 40 °C, eluted with an isocratic water–methanol system. The elevated temperature reduces the column backpressure and allows using higher flow rates, making the analysis more rapid. Since the molar absorption coefficient of PEGn-Ts does not depend significantly on the chain length at the wavelength λ > 266 nm (Fig. S1†), RP-HPLC analysis with UV detection is considered as quantitative for PEGn-Ts polydispersity determination.
The analysis showed that in general PEGn-Ts contain a small amount of PEG(n−1)-Ts and PEG(n+1)-Ts (Fig. 1c, d and S2a–f†). Additionally, traces of PEG(n−4)-Ts were found as a result of either incomplete Williamson ether synthesis reaction or PEG4 being not completely washed away after the reaction.¶ The monodispersity of the product decreases slowly with the chain length, and seems to be less affected by base-induced depolymerisation than by the purity of the used PEG4 (Tables 1 and S1†). The oligomer purity of the monotosylates synthesized by the chromatography-free procedure was determined by HPLC as 98.7%, 98.2% and 97.0% for PEG8-Ts, PEG12-Ts and PEG16-Ts, respectively.
| Compound | MALDI-La | MALDI-Rb | RP-HPLCc |
|---|---|---|---|
| a Analysis in the linear mode. b Analysis in the reflectron mode. c Calculated on the basis of UV absorbance at 280 nm. All values are expressed in molar %. For more details, see the ESI. | |||
| PEG8-Ts (5) | 98.9% | 99.4% | 98.7% |
| PEG12-Ts (8) | 98.3% | 99.3% | 98.2% |
| PEG16-Ts (11) | 98.3% | 99.1% | 97.0% |
Surprisingly, when we compared the purity values obtained with HPLC analysis with those measured by mass spectrometry, it appeared that the results are not identical. Spectra recorded with MALDI-TOF in a reflectron mode showed a significantly lower amount of minor oligomers, i.e. significantly overestimated the purity (Tables 1, S1 and Fig. S3†). For instance, PEG16-Ts, showing above 99% purity by MALDI-TOF in reflectron mode, had an actual purity of 97% (as determined with HPLC). Spectra recorded with the MALDI-TOF spectrometer in a linear mode showed relatively good agreement with the results from HPLC analysis for shorter (n = 8, 12) oligomers, while for PEG16-Ts the difference was significant. Analyses performed with two different ESI-TOF MS spectrometers with the same ion source settings yielded completely different, inconsistent results. This suggests that ESI-TOF MS may be very sensitive to certain measurement conditions and therefore not a reliable source of information about oligomer purity. This conclusion is in agreement with one of the recent studies.40 However, MALDI-TOF seems to be unsuitable for the monodispersity analysis as well. Although HPLC methods have been used for analysis and even preparative scale separations of PEGs by the chemical industry, in all the reports published recently in scientific journals, mass spectrometry was routinely employed for monodispersity analysis.
To show the versatility of PEGn-Ts as intermediates for PEG-based heterobifunctional monodisperse compounds, several useful PEG derivatives were prepared from tosylate 5 in few steps (Scheme 3).|| We confirmed that both PEG termini can be orthogonally modified with various functional groups. The hydroxy group was reacted with t-butyl acrylate through oxo-Michael addition, yielding the corresponding t-butyl propionate-PEG ester (12). This process was also done without chromatographic purification, giving the product in high yield. The ester group was further reduced to aldehyde (13), reactive towards amino groups, or alternatively hydrolysed to propionic-PEG acid (14), suitable for activation with the N-hydroxysuccinimide group (NHS). The hydroxy group was also modified with divinyl sulfone, affording vinyl sulfone-PEG (15), showing similar reactivity towards thiol groups as widely used maleimide-PEG, although resistant to hydrolysis.44–46 All the products retained the original p-toluenesulfonyl group on the opposite end of the chain, being suitable for further derivatisation.The p-toluenesulfonyl group can be transformed in numerous ways through SN2 reactions. Here we showed three simple examples of such modifications. The tosylate was converted into azide-PEG (16), useful for 1,3-dipolar cycloaddition, and further into amine-PEG (17), which can be transformed into maleimide-PEG. Alternatively, through the corresponding thioacetate we obtained thiol-PEG (18), suitable for surface modification.
 | ||
| Scheme 3 Synthesis of heterobifunctional PEG derivatives obtained from octa(ethylene glycol) mono-p-toluenesulfonate 5. | ||
Because the only source of polydispersity in derivatives 12–18 is tosylate 5, their oligomeric purity should not be lower than that of 5. It may be further improved during column chromatographic purification of the derivatives.
Conclusions
Here we report an efficient synthesis of monodisperse oligo(ethylene glycol) mono-p-toluenesulfonates, featuring both no chromatographic purification and high final purity. To the best of our knowledge, this is the first chromatography-free synthetic method yielding asymmetrically substituted monodisperse PEGs. Our approach surpasses previously reported methods in terms of experimental simplicity, cost efficiency and overall reaction yields. An HPLC-based quantitative analytical method is used to confirm the high monodispersity of the products and is compared with conventional MS-based methods, showing the MS analyses to be inaccurate. We also show that oligomeric PEGn-Ts can be orthogonally functionalized into various heterobifunctional derivatives bearing groups common in bioconjugate chemistry.Experimental
Tetra(ethylene glycol) (99%, item no. 110175) was purchased from Sigma-Aldrich and used without further purification. Dry THF was passed through the PPT Glass Contour Solvent Purification System before using.
1H and 13C NMR spectra were recorded at 298 K in CDCl3 at 400 MHz and 101 MHz, respectively, with a Bruker Avance III 400 spectrometer. MALDI-TOF MS analyses were performed with a Bruker Autoflex Speed spectrometer with a 355 nm laser in a linear positive or reflectron positive mode. The samples were prepared on a ground steel target plate with an α-cyano-4-hydroxycinnamic acid (CHCA) matrix by mixing 1 μL of saturated matrix solution in acetonitrile and 1 μL of the analyte solution in water or chloroform (ca. 1% w/w; molar ratio sample–matrix ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). ESI-TOF MS analyses were performed with a Bruker MicrOTOF-Q II spectrometer in a positive mode with source voltage 4.5 kV from the methanolic solution (ca. 1 μM). MS data were analysed by using an mMass open source tool.47
5). ESI-TOF MS analyses were performed with a Bruker MicrOTOF-Q II spectrometer in a positive mode with source voltage 4.5 kV from the methanolic solution (ca. 1 μM). MS data were analysed by using an mMass open source tool.47
HPLC analyses were carried out with a JASCO LC-2000 Plus system equipped with Nacalai Cosmosil 5C18-AR-II, 4.6 × 150 mm column at 40 °C. The mobile phase was composed of methanol–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with 5 mM ammonium acetate. For every analysis 5 μL of 0.3% (w/w) sample was injected and eluted at a rate of 0.8 mL min−1. UV detector wavelengths were set at 254 and 280 nm. UV spectra were recorded on a JASCO V-650 spectrophotometer in methanol–water 1
1) with 5 mM ammonium acetate. For every analysis 5 μL of 0.3% (w/w) sample was injected and eluted at a rate of 0.8 mL min−1. UV detector wavelengths were set at 254 and 280 nm. UV spectra were recorded on a JASCO V-650 spectrophotometer in methanol–water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 0.83 mM sample concentration.
1 at 0.83 mM sample concentration.
Acknowledgements
We are grateful to Dr Eui-Chul Kang and Mr Tomoyuki Ohtake (NOF Corporation) for their support in the chromatographic analyses. This work was partially supported by the Ministry of Education, Culture, Sports, Science and Technology in Japan (MEXT), Grants-in-Aid for Scientific Research on Innovative Areas “Spying minority in biological phenomena” (no. 3306, 23115003, KK) and Scientific Research C (26410170, TM), JST PRESTO “Molecular technology and creation of new functions”, and the Management Expenses Grants for National Universities Corporations.Notes and references
- C. Kojima, K. Kono, K. Maruyama and T. Takagishi, Bioconjugate Chem., 2000, 11, 910–917 CrossRef CAS PubMed.
- Y. Pang, J. Liu, J. Wu, G. Li, R. Wang, Y. Su, P. He and X. Zhu, Bioconjugate Chem., 2010, 21, 2093–2102 CrossRef CAS PubMed.
- A. L. Acton, C. Fante, B. Flatley, S. Burattini, I. W. Hamley, Z. Wang, F. Greco and W. Hayes, Biomacromolecules, 2013, 14, 564–574 CrossRef CAS PubMed.
- S. Hocine and M.-H. Li, Soft Matter, 2013, 9, 5839–5861 RSC.
- T. Shima, T. Muraoka, N. Hoshino, T. Akutagawa, Y. Kobayashi and K. Kinbara, Angew. Chem., Int. Ed., 2014, 53, 7173–7178 CrossRef CAS PubMed.
- H. Kamata, Y. Akagi, Y. Kayasuga-Kariya, U. Chung and T. Sakai, Science, 2014, 343, 873–875 CrossRef CAS PubMed.
- T. Muraoka, T. Endo, K. V. Tabata, H. Noji, S. Nagatoishi, K. Tsumoto, R. Li and K. Kinbara, J. Am. Chem. Soc., 2014, 136, 15584–15595 CrossRef CAS PubMed.
- T. Muraoka and K. Kinbara, Chem. Commun., 2016, 52, 2667–2678 RSC.
- O. Tirosh, Y. Barenholz, J. Katzhendler and A. Priev, Biophys. J., 1998, 74, 1371–1379 CrossRef CAS PubMed.
- C. C. A. Ng, A. Magenau, S. H. Ngalim, S. Ciampi, M. Chockalingham, J. B. Harper, K. Gaus and J. J. Gooding, Angew. Chem., Int. Ed., 2012, 51, 7706–7710 CrossRef CAS PubMed.
- S. C. Rizzi and J. A. Hubbell, Biomacromolecules, 2005, 6, 1226–1238 CrossRef CAS PubMed.
- G. Elia, Proteomics, 2008, 8, 4012–4024 CrossRef CAS PubMed.
- Y. Lee and P. Famouri, Nano Lett., 2013, 13, 3775–3782 CrossRef CAS PubMed.
- F. M. Veronese, Biomaterials, 2001, 22, 405–417 CrossRef CAS PubMed.
- M. J. Roberts, M. D. Bentley and J. M. Harris, Adv. Drug Delivery Rev., 2002, 54, 459–476 CrossRef CAS PubMed.
- K. Knop, R. Hoogenboom, D. Fischer and U. S. Schubert, Angew. Chem., Int. Ed., 2010, 49, 6288–6308 CrossRef CAS PubMed.
- G. Pasut and F. M. Veronese, J. Controlled Release, 2012, 161, 461–472 CrossRef CAS PubMed.
- J. Herzberger, K. Niederer, H. Pohlit, J. Seiwert, M. Worm, F. R. Wurm and H. Frey, Chem. Rev., 2016, 116, 2170–2243 CrossRef CAS PubMed.
- G. T. Hermanson, in Bioconjugate Techniques, Elsevier, 2013, pp. 787–838 Search PubMed.
- E. M. D. Keegstra, J. W. Zwikker, M. R. Roest and L. W. Jenneskens, J. Org. Chem., 1992, 57, 6678–6680 CrossRef CAS.
- A. Zada, Y. Avny and A. Zilkha, J. Surfactants Deterg., 2001, 4, 163–166 CrossRef CAS.
- B. A. Burkett and T. H. Chan, Synthesis, 2004, 1007–1010 CAS.
- S. A. Ahmed and M. Tanaka, J. Org. Chem., 2006, 71, 9884–9886 CrossRef CAS PubMed.
- D. Niculescu-Duvaz, J. Getaz and C. J. Springer, Bioconjugate Chem., 2008, 19, 973–981 CrossRef CAS PubMed.
- D. Lumpi, C. Braunshier, C. Hametner, E. Horkel, B. Zachhuber, B. Lendl and J. Fröhlich, Tetrahedron Lett., 2009, 50, 6469–6471 CrossRef CAS.
- C. M. Gothard and B. A. Grzybowski, Synthesis, 2012, 717–722 CAS.
- M. S. Thompson, T. P. Vadala, M. L. Vadala, Y. Lin and J. S. Riffle, Polymer, 2008, 49, 345–373 CrossRef CAS.
- G. T. Hermanson, in Bioconjugate Techniques, Elsevier, 3rd edn, 2013, pp. 299–339 Search PubMed.
- G. T. Hermanson, in Bioconjugate Techniques, Elsevier, 3rd edn, 2013, pp. 867–920 Search PubMed.
- A. Bouzide and G. Sauve, Org. Lett., 2002, 4, 2329–2332 CrossRef CAS PubMed.
- F. A. Loiseau, K. K. Hii and A. M. Hill, J. Org. Chem., 2004, 69, 639–647 CrossRef CAS PubMed.
- B. La Ferla, C. Zona and F. Nicotra, Synlett, 2009, 2325–2327 CrossRef CAS.
- A. C. French, A. L. Thompson and B. G. Davis, Angew. Chem., Int. Ed., 2009, 48, 1248–1252 CrossRef CAS PubMed.
- Y. Li, Q. Guo, X. Li, H. Zhang, F. Yu, W. Yu, G. Xia, M. Fu, Z. Yang and Z.-X. Jiang, Tetrahedron Lett., 2014, 55, 2110–2113 CrossRef CAS.
- Q. Zhang, H. Ren and G. L. Baker, Tetrahedron Lett., 2014, 55, 3384–3386 CrossRef CAS.
- G. Székely, M. Schaepertoens, P. R. J. Gaffney and A. G. Livingston, Polym. Chem., 2014, 5, 694–697 RSC.
- G. Székely, M. Schaepertoens, P. R. J. Gaffney and A. G. Livingston, Chem. – A Eur. J., 2014, 20, 10038–10051 CrossRef PubMed.
- H. Zhang, X. Li, Q. Shi, Y. Li, G. Xia, L. Chen, Z. Yang and Z.-X. Jiang, Angew. Chem., Int. Ed., 2015, 54, 3763–3767 CrossRef CAS PubMed.
- Y. Li, X. Qiu and Z.-X. Jiang, Org. Process Res. Dev., 2015, 19, 800–805 CrossRef CAS.
- K. Maranski, Y. G. Andreev and P. G. Bruce, Angew. Chem., Int. Ed., 2014, 53, 6411–6413 CrossRef CAS PubMed.
- M. Ouchi, Y. Inoue, Y. Liu, S. Nagamune, S. Nakamura, K. Wada and T. Hakushi, Bull. Chem. Soc. Jpn., 1990, 63, 1260–1262 CrossRef CAS.
- N. Boden, R. J. Bushby, S. Clarkson, S. D. Evans, P. F. Knowles and A. Marsh, Tetrahedron, 1997, 53, 10939–10952 CrossRef CAS.
- K. Shimada, R. Nagahata, S. Kawabata, S. Matsuyama, T. Saito and S. Kinugasa, J. Mass Spectrom., 2003, 38, 948–954 CrossRef CAS PubMed.
- M. Morpurgo, F. M. Veronese, D. Kachensky and J. M. Harris, Bioconjugate Chem., 1996, 7, 363–368 CrossRef CAS PubMed.
- B. D. Mather, K. Viswanathan, K. M. Miller and T. E. Long, Prog. Polym. Sci., 2006, 31, 487–531 CrossRef CAS.
- J. Morales-Sanfrutos, J. Lopez-Jaramillo, M. Ortega-Muñoz, A. Megia-Fernandez, F. Perez-Balderas, F. Hernandez-Mateo and F. Santoyo-Gonzalez, Org. Biomol. Chem., 2010, 8, 667–675 CAS.
- M. Strohalm, M. Hassman, B. Kosata and M. Kodicek, Rapid Commun. Mass Spectrom., 2008, 22, 905–908 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental discussion and monodispersity analysis; synthetic procedures; 1H and 13C NMR spectra; ESI-MS spectra. See DOI: 10.1039/c6py00127k |
| ‡ Although functionalized PEGs are not PEGs in strict meaning, for the sake of simplicity this name is used throughout the text. |
| § The yields were calculated based on the trityl chloride amount, the molar limiting reactant that also generated the largest fraction of the total material cost. |
| ¶ The source of PEG(n+1)-Ts impurities is PEG5 present in commercial PEG4. Because short PEGs are produced by polymerization, followed by fractional distillation to isolate individual oligomers, commercial products are contaminated with traces of shorter and longer oligomers. |
| || Some of the derivatives were prepared without the use of chromatographic columns. However, due to the nature of some reactions employed in derivatisation, especially ester reduction to aldehyde, in some cases chromatographic purification was necessary. |
| This journal is © The Royal Society of Chemistry 2016 |