Kinetics of bulk photo-initiated copper(i)-catalyzed azide–alkyne cycloaddition (CuAAC) polymerizations†
Abstract
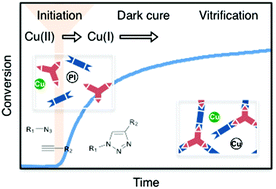
Photoinitiation of polymerizations based on the copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) reaction enables spatio-temporal control and the formation of mechanically robust, highly glassy photopolymers. Here, we investigated several critical factors influencing photo-CuAAC polymerization kinetics via systematic variation of reaction conditions such as the physicochemical nature of the monomers; the copper salt and photoinitiator types and concentrations; light intensity; exposure time and solvent content. Real time Fourier transform infrared spectroscopy (FTIR) was used to monitor the polymerization kinetics in situ. Six different di-functional azide monomers and four different tri-functional alkyne monomers containing either aliphatic, aromatic, ether and/or carbamate substituents were synthesized and polymerized. Replacing carbamate structures with ether moieties in the monomers enabled an increase in conversion from 65% to 90% under similar irradiation conditions. The carbamate results in stiffer monomers and higher viscosity mixtures indicating that chain mobility and diffusion are key factors that determine the CuAAC network formation kinetics. Photoinitiation rates were manipulated by altering various aspects of the photo-reduction step; ultimately, a loading above 3 mol% per functional group for both the copper catalyst and the photoinitiator showed little or no rate dependence on concentration while a loading below 3 mol% exhibited 1st order rate dependence. Furthermore, a photoinitiating system consisting of camphorquinone resulted in 60% conversion in the dark after only 1 minute of 75 mW cm−2 light exposure at 400–500 nm, highlighting a unique characteristic of the CuAAC photopolymerization enabled by the combination of the copper(I)'s catalytic lifetime and the nature of the step-growth polymerization.

 Please wait while we load your content...
Please wait while we load your content...