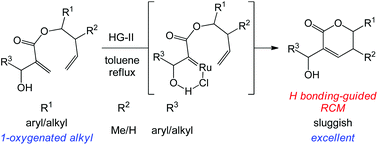
An efficient hydrogen bonding-guided ring-closing metathesis (RCM) reaction of sterically demanding homoallyl 2-(hydroxymethyl)acrylates catalyzed by the Hoveyda–Grubbs 2nd generation catalyst was developed and the reaction mechanism was explored. Adding a substituent to the hydroxymethyl group in this scaffold resulted in a class of challenging RCM substrates, although usable yields could be obtained. However, substrates bearing a 1-oxygenated alkyl group on the homoallylic carbon gave excellent RCM yields, providing a practical solution. Experimental and computational evidence indicated an unusual directing effect of OH⋯Cl hydrogen bonding between the substrate and Ru catalyst, which guides Ru to interact with the electron-deficient, more hindered acrylic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and thus triggers the RCM process.
C bond and thus triggers the RCM process.