Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent advances in the synthesis and application of fluorescent α-amino acids
Alexander H.
Harkiss
and
Andrew
Sutherland
*
WestCHEM, School of Chemistry, The Joseph Black Building, University of Glasgow, Glasgow G12 8QQ, UK. E-mail: Andrew.Sutherland@glasgow.ac.uk
First published on 30th August 2016
Abstract
Fluorescence spectroscopy has become a powerful technique for probing a range of complex biological processes including enzyme mechanisms and protein–protein interactions. While the application of this technique uses a number of strategies, many of these rely on the use of fluorescent α-amino acids. This review highlights the recent synthetic methods developed for the incorporation of highly conjugated chromophores into the side-chain of α-amino acids and the application of these compounds as probes for imaging in medicine and biology. In particular, the design and synthesis of α-amino acids bearing coumarin, flavone and polyaromatic derived chromophores is described.
1. Introduction
In the last 20 years, fluorescence spectroscopy has played a key role as an analytical and diagnostic tool in many areas of fundamental and applied biomolecular science.1 Significant developments in research fields such as molecular and cellular biology, biophysics, biotechnology and medicine have been accelerated due to the application of emerging fluorescent-based experiments.1,2 This is in part due to the high sensitivity, selectivity and fast response time of this technique, as well as the relative ease in tuning the photophysical properties of small molecular probes.As proteins are crucial for the majority of cellular functions, the investigation of these processes, which involve enzyme mechanisms and protein–protein interactions, can be achieved in combination with fluorescent spectroscopy using a number of strategies. These include expressing the protein of interest with a naturally occurring fluorescent protein (e.g. green fluorescent protein, GFP)3 or using naturally occurring fluorescent α-amino acids within a protein, such as tyrosine or tryptohan.4 However, there are limitations to such approaches. For example, attachment of fluorescent proteins can alter the stability and functionality of their fusion partner, while the use of intrinsic fluorescent α-amino acids relies on a relative abundance within the protein and multiple residues in different environments can complicate the spectroscopy. One of the main alternative approaches involves the design and application of unnatural fluorescent α-amino acid analogues.4–9 These compounds can be readily accessed using standard methods for the asymmetric synthesis of α-amino acids.10 The photophysical properties of unnatural fluorescent α-amino acids can be tuned for a particular application and they can be incorporated into protein structures with a high level of precision using conventional solid phase peptide synthesis (SPPS), expressed protein ligation (EPL) or unnatural amino acid mutagenesis.8 Unnatural fluorescent α-amino acids have been used in wide-ranging applications and recent examples include the study of protein dynamics and local conformation changes in enzymes such as dihydrofolate reductase (DHFR) and the E. coli glutamate binding protein.11–13 These types of probes have also been used for the investigation of peptidoglycan synthesis and to monitor subsequent bacterial growth.14
Due to the significant developments in the design and application of unnatural fluorescent α-amino acids and peptides over the last two decades, several reviews have been published in this area.4–9 For example, in 2009, Katritzky and Narindoshvili described the specific structural features required for the use of fluorescent α-amino acids as effective molecular probes,6 while more recently Krueger and Imperiali have reviewed the incorporation of unnatural fluorescent α-amino acids into peptides and proteins and their application in various chemical biology studies.8 The aim of this review is to highlight the recent synthetic methods and strategies that have been developed for the preparation of fluorescent α-amino acids incorporating side-chain chromophores. The use of these compounds as biosensors and molecular probes is also described. The review has been organised based on the structure of the chromophore side-chain and this includes compounds derived from natural α-amino acids and well-established coumarin, fluorescein and polyaromatic-type chromophores.
2. Fluorescent analogues of natural α-amino acids
Naturally occurring α-amino acids bearing aromatic side-chains, such as L-phenylalanine (1), L-tyrosine (2) and L-tryptophan (3) (Fig. 1) have been used as intrinsic fluorescent probes to study phenomena such as protein dynamics.15 The use of naturally occurring α-amino acids for fluorescent imaging minimises the conformational perturbations of protein structure and loss of stability sometimes observed when using structurally different unnatural α-amino acids. Despite these advantages, their general application in fluorescent studies has been limited by poor optical properties. For example, phenylalanine has both low quantum yield and molar extinction coefficient, while tyrosine lacks sensitivity to environmental changes such as polarity. For these reasons, a number of novel fluorescent α-amino acids have been developed based on fine-tuning the structures and optical properties of naturally occurring α-amino acids.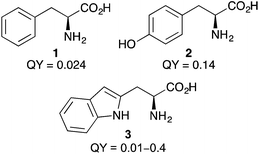 | ||
| Fig. 1 Structures and quantum yields (QY)4 of L-phenylalanine (1), L-tyrosine (2) and L-tryptophan (3). | ||
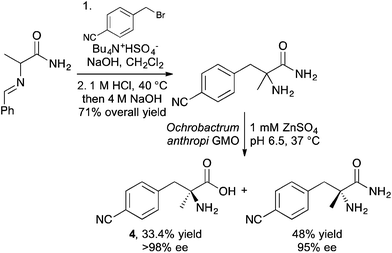
A p-cyano-derivative of L-phenylalanine has been synthesised and used to study peptide–membrane interactions.16 The key step of the synthesis involved a phase transfer catalysed alkylation of a benzylidene protected alanine amide (Scheme 1). Following hydrolysis of the benzylidene protecting group, the racemic amino amide was resolved using the genetically modified organism Ochrobactrum anthropi which gave the desired L-phenylalanine derivative 4 in >98% enantiomeric excess. The incorporation of this α-methyl derivative into the decapeptide trichogin GA IV resulted in retention of helical conformation. Using the optical properties of the p-cyanophenyl moiety, the modified peptide was shown to have fluorescence emission around 295–305 nm and this was exploited to investigate the interaction of this modified lipopeptaibol with model membranes.
L-Tyrosine derivatives tend to have significantly higher quantum yields than the corresponding L-phenylalanine derivatives and as such, new fluorescent derivatives have been prepared17 and used in applications such as the biosensing of hydrogen peroxide.18
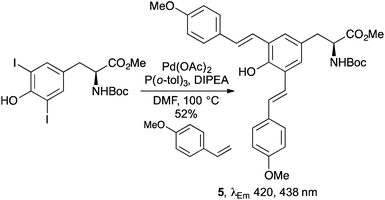
A series of stimuli responsive fluorescent α-amino acids have been designed based on extending the π-conjugation of L-tyrosine.19 Compounds such as the bis-styryl analogue 5 were prepared using a palladium-catalysed double Mizoroki–Heck reaction of a 3,5-diiodo-L-tyrosine derivative with 4-methoxystyrene (Scheme 2). A pyridine derivative from this series displayed distinct red, green and blue emission spectra by control of the solution pH, while the incorporation of bis-styryl analogue 5 into a cell-penetrating peptide and incubation with two different cell lines showed that these compounds could be used as intrinsic labels for fluorescent imaging of cells.
Of the three naturally occurring fluorescent α-amino acids, L-tryptophan has the most favourable photophysical properties, such as more intense brightness. For these reasons, a wide range of indole variants such as azulene and benzothiophenyl have been used to mimic the size and polarity of the L-tryptophan side-chain, resulting in novel fluorescent α-amino acids.4
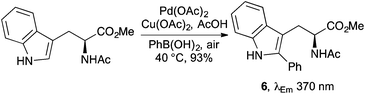
More recently, new methodology has been developed that allows C-2 substitution of the indole ring leading to novel fluorescent L-tryptophan analogues.20,21 For example, Chen and co-workers prepared a fluorescent L-tryptophan bearing a 1,2,3-triazole at C-2 using a hydrogen-bond mediated coupling reaction,20 while the group of Fairlamb produced a series of novel, highly fluorescent C-2 arylated L-tryptophan analogues and L-tryptophan-containing peptides using a palladium-mediated direct C–H bond functionalisation (Scheme 3).21 The emission wavelength of these C-2 aryl compounds were found to be red-shifted relative to L-tryptophan, with phenyl analogue 6 exhibiting the largest fluorescence intensity.
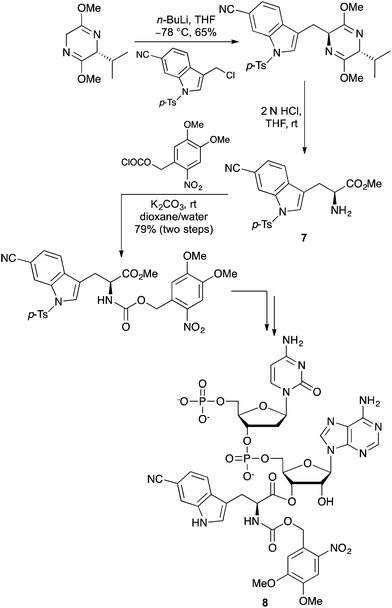
Hecht and co-workers have reported a series of highly fluorescent L-tryptophan analogues incorporating additional heteroatoms, heterocycles or conjugated substituents within the indole ring system.22 Many of these analogues were found to be significantly brighter and red-shifted in fluorescence emission compared to L-tryptophan. A general asymmetric synthetic route to these compounds was developed by alkylation of the Schöllkopf bis-lactim ether with an indole derivative to give the adducts in high diastereoselectivity. In the case of the 6-cyano analogue 7 (Scheme 4), this was converted to dinucleotide 8 and used to activate a suppressor tRNA transcript for efficient incorporation of the amino acid into two different positions of E. coli DHFR.22c Fluorescent imaging of the 6-cyanotryptophan in DHFR could be performed selectively in the presence of tryptophan analogues and was also shown to form an efficient Förster resonance energy transfer (FRET) pair with a coumarin derived glycine.
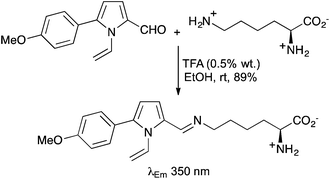
The side-chains of other naturally occurring α-amino acids such as aspartic acid,23 lysine24 and serine25 have been labelled with various chromophores, resulting in the preparation of novel fluorescent α-amino acids. For example, in a direct and highly regioselective approach, L-lysine has been condensed with a range of 1-vinylpyrrole-2-carbaldehydes under mild conditions to give the corresponding Schiff bases in high yields (Scheme 5).24c These compounds were shown to fluoresce in the UV-visible region (350–382 nm).
3. Coumarin derived α-amino acids
2H-Chromen-2-ones, commonly known as coumarins or benzopyranones, are found in a wide range of natural products, pharmaceuticals and new materials.26 This chromophore has also been widely used in optical imaging due to this ring system having high quantum yields, an extended spectropscopic range, photostability and general solubility in a wide range of solvents.5,6 For these reasons, many synthetic methods have been developed to incorporate the coumarin motif and more specifically, 7-methoxy or 6,7-dimethoxy analogues into the side-chain of α-amino acids.27 In 2004, Garbay and co-workers reported a short synthetic approach for the preparation of optically active coumarin derived α-amino acids using a von Pechmann condensation reaction of a β-keto ester with a phenol.28 The side-chains of L-aspartic acid (Scheme 6) and L-glutamic acid were initially activated with carbonyldiimidazole and then reacted with the magnesium salt of monoethyl malonic acid to give the corresponding β-keto esters. Hydrogenolysis led to the removal of the protecting groups and this was followed by the von Pechmann reaction, performed in the presence of methanesulfonic acid. Using 3-methoxyphenol, allowed the synthesis of L-(7-methoxycoumarin-4-yl)alanine (9), a commonly used imaging agent in 71% yield.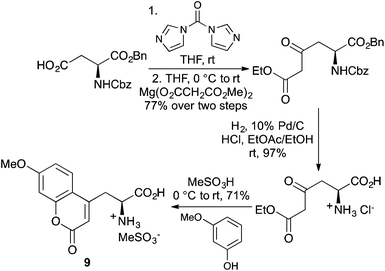 | ||
| Scheme 6 Application of the von Pechmann condensation reaction for the preparation of coumarin derived α-amino acids. | ||
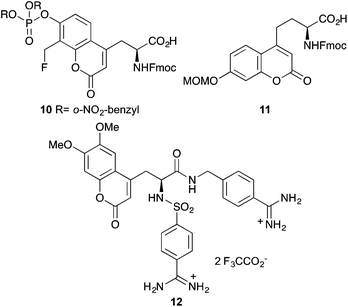
More recently, this approach has been used to prepare various functionalised coumarin derived α-amino acids for biological imaging.29–31 A phosphate derivative 10 (Fig. 2) was prepared as a mimic of phosphotyrosine and following incorporation into cell penetrating peptides, was used to study the activity of protein tyrosine phosphatases.29 Martin and co-workers developed an optimised protocol based on the von Pechmann reaction for the efficient and scalable synthesis of protected coumarin derived ethylglycine 11.30 This was found to be an excellent building block for SPPS and following incorporation into truncated peptides of HIV-Tat, was shown to be effective at HeLa cell imaging. Using the Garbay approach, Häuβler and Gütschow prepared a fluorescent bisbenzamidine coumarin derivative 12, which has an emission maximum at 432 nm.31 The authors propose that this compound could find application in the study of minor grooves of adenine/thymine-rich double-strand DNA.
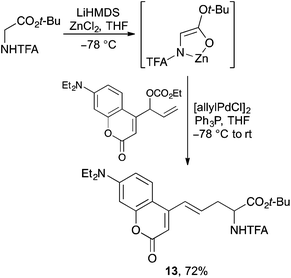
New synthetic methodology has also been developed for the preparation of both known and novel coumarin derived α-amino acids.32–34 The groups of Braun32 and Wang33 have both shown that (7-hydroxycoumarin-4-yl)ethylglycine can be efficiently prepared by the reaction of a glycine-enolate equivalent with a suitably activated coumarin derived electrophile. In a similar manner, Kazmaier and co-workers prepared a series of novel α-amino acids bearing a coumarin side-chain by the palladium-catalysed allylic alkylation of coumarin activated allylic carbonates with a zinc chelated glycine-enolate (Scheme 7).34 Compounds such as 13 were prepared in good yield with the double bond in conjugation with the coumarin and formed exclusively as the E-isomer.
4. Fluorescent α-amino acids incorporating other established chromophores
A range of other commonly used fluorophores, such as fluorescein, benzothiadiazole, xanthone and BODIPY have been used to label non-emissive, unnatural α-amino acids and peptides for application in biological imaging.14b,35,36 A good example of this is the work reported by the Xie group who showed that various alkyne-derived chromophores could be attached to azido α-amino acids using click chemistry.37 In an extension of this work, the use of a ruthenium-catalysed cycloaddition reaction of bis-alkyne substituted benzothiadiazole motifs with azidoalanine derivatives gave crosslinked α-amino acids in good yields (Scheme 8).37c An investigation of the photophysical effects of compounds such as 14 showed that conjugating 1,2,3-triazoles with the benzothiadiazole group gave longer excitation and emission wavelengths while maintaining high fluorescence quantum yields.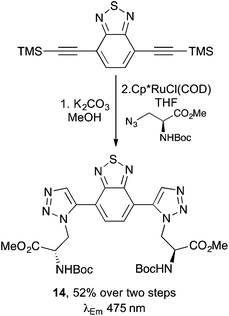 | ||
| Scheme 8 Synthesis of cross-linked α-amino acid 14 using a ruthenium-catalysed cycloaddition reaction. | ||
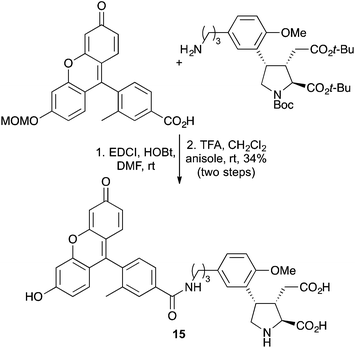
Other standard coupling techniques such as amide bond formation have been used to attach chromophores to the side chains of unnatural α-amino acids.38 The fluorescein photophore, Tokyo-Green was coupled to an aminopropyl analogue of the kainoid MFPA using a combination of EDCI and HOBt (Scheme 9). Following deprotection, the resulting fluorescent adduct 15, was used to probe the ionotropic glutamate receptor in mice.
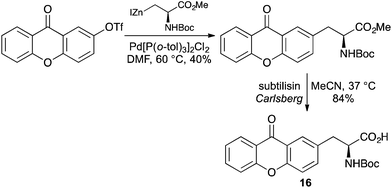
As well as simply labelling the side-chain of an α-amino acid with an established chromophore, many fluorescent α-amino acids have been generated by using the chromophore as the entire side-chain. This has the advantage of minimising steric pertubations when incorporated into peptides and proteins. For example, preparation of an xanthone-alanine derivative 16 was achieved by the palladium-catalysed coupling of an xanthone triflate with an alanine derived organozinc reagent (Scheme 10).39 Enzymatic hydrolysis of the ester gave the optically pure Boc-protected amino acid, which showed typical xanthone fluorescent characteristics.
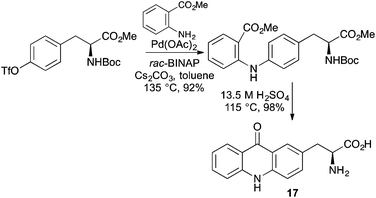
The benefits of incorporating a chromophore directly within the side-chain of an α-amino acid is exemplified by L-acridon-2-ylalanine 17, which is small enough to be utilised in ribosomal biosynthesis.40 A highly efficient scalable synthetic route was developed from L-tyrosine using a Buchwald–Hartwig type coupling of a triflate derivative with O-methyl anthranilate (Scheme 11).40,41 Treatment of the coupled product with sulfuric acid resulted in both an intramolecular Friedel–Crafts acylation and deprotection of the amino acid. An aminoacyl tRNA enzyme evolved from Methanococcus janaschii tyrosine synthetase was effective for the milligram synthesis of L-acridon-2-ylalanine-labelled proteins. Characterisation of the photophysical properties of these proteins, demonstrated that 17 was an effective partner in FRET interactions and could be used to monitor peptide binding and protein folding.
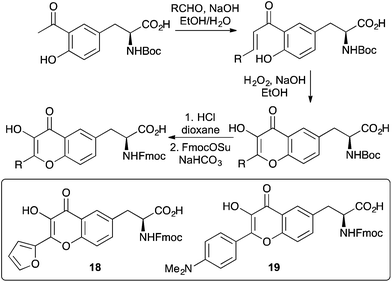
Flavone containing α-amino acids have also been prepared as fluorescent probes for a number of applications.42 A general synthesis of 3-hydroxyflavone α-amino acids was developed from an L-tyrosine derivative (Scheme 12). Aldol condensation gave a chalcone intermediate that was then subjected to an Algar–Flynn–Oyamada reaction forming the 3-hydroxyflavone motif.43 These compounds were then typically converted to the Fmoc-derivatives for SPPS. Furan analogue 18, which displays dual emission was incorporated into the zinc finger domain of the HIV-1 nucleocapsid protein in place of a tryptophan residue.42a Despite this change, the folding of the protein was preserved and was used to investigate binding to oligonucleotides. The advantage of placing the chromophore in close proximity to the peptide main-chain was demonstrated with the push–pull 4-dimethylaminophenyl analogue 19.42b This dual fluorescent dye was incorporated into the membrane-active peptide, melittin and used to probe the orientation of the peptide in lipid bilayers. The proximity of the fluorophore in combination with its highly sensitive dual emission properties resulted in detection of the peptide at a much deeper level in the bilayer.
5. Aminonaphthyl and aminophthalimide-type fluorescent α-amino acids
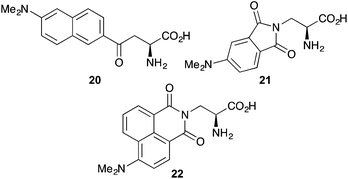
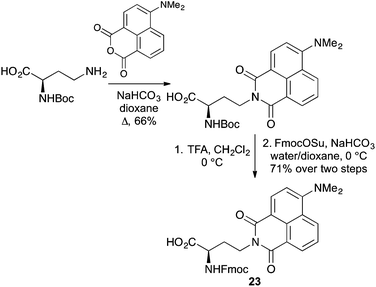
Over the last two decades α-amino acids bearing various push–pull chromophores such as aminonaphthyl and aminophthalimide have been developed as highly environmentally sensitive probes for biological processes (Fig. 3). The charge transfer dye, dimethylaminonaphthyl 20, simultaneously developed by the Imperiali and Cohen groups, was used to study peptide–protein interactions associated with the S-peptide of RNase S,44 as well as the electrostatic character of the B1 domain of streptococcal protein G.45 The dimethylaminophthalimide α-amino acid 21, as a component of a short peptide sequence, was shown to be an excellent reporter for binding with a 14-3-3 protein.46 A 6-fold increase in fluorescence intensity and a 40 nm shift of the emission maximum was observed on binding to the 14-3-3 protein. More recently, the Imperiali group reported a new member of the dimethylaminophthalimide family of fluorophores, the 4-N,N-dimethylamino-1,8-naphthalimide analogue 22.47 This solvatochromic α-amino acid was found to have a number of advantages over other members of this family, including greater chemical stability and longer wavelength of excitation. A demonstration of its use to monitor protein–protein interactions by incorporation into a peptide recognised by calcium-activated calmodulin showed a 900-fold increase in fluorescence emission on binding.Modified versions of this family of fluorescent α-amino acids have recently been reported. A chain-extended D-configured version of 22 has been prepared and incorporated into peptides.48 The synthesis of this novel analogue, which is typical for most phthalimide and naphthalimide compounds involved the condensation of an anhydride with a suitably protected α-amino acid with an amine side chain (Scheme 13). The amino protecting group was replaced with Fmoc to give 23 and this was incorporated into short peptides using SPPS.
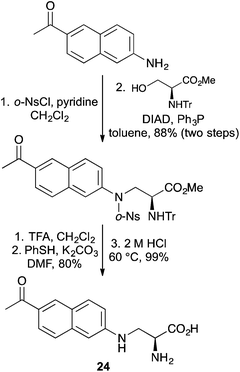
The fluorescent unnatural amino acid L-3-(6-acetylnaphthalen-2-ylamino)-2-aminopropanoic acid (24) has been genetically incorporated into various proteins to study protein–ligand and protein–protein interactions in Saccharomyces cerevisiae and mammalian cells.12,49,50 The relatively small size of 24 and the close proximity of the chromophore to the protein backbone means it is a good substitute for natural α-amino acids, with minimal perturbation to protein conformation. A new synthesis of 24 has been reported by Xiang and Wang, who used a Fukuyama–Mitsunobu reaction to implement the key step (Scheme 14).51 This allowed the efficient coupling of a 2-aminonaphthyl unit with a serine derivative. Stepwise deprotection of the various protecting groups under mild conditions gave 24 in high optical purity.
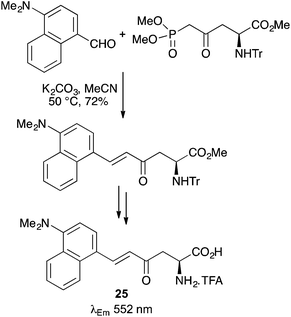
Novel fluorescent α-amino acids bearing aminonaphthyl side-chains have been prepared using strategies such as click-chemistry to couple the chromophore with the amino acid core.52 Other approaches include the use of a mild Horner–Wadsworth–Emmons (HWE) reaction between an L-aspartic acid derived β-keto phosphonate ester and 4-dimethylamino-1-naphthaldehyde (Scheme 15).53 Following deprotection, E-enone 25 displayed solvatochromic properties and an emission maximum at 552 nm in water.
6. Fluorescent polyaromatic α-amino acids
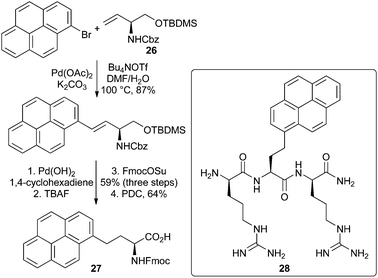
Fluorescent α-amino acids with conjugated polyaromatic side-chains tend to have similar spectroscopic properties (e.g. quantum yields) in polar and apolar environments. While this limits the application of these compounds as reporting probes, they can be used as fluorescent tags.A range of general methods have been developed for the synthesis of this class of fluorescent α-amino acid that involve a coupling reaction between an activated amino acid core and a suitably functionalised polyaromatic group.54 A number of approaches utilise dehydroalanine derivatives in combination with either conjugate addition55,56 or Diels–Alder reactions.57 A Heck reaction between a vinylglycinol derivative and polyaromatic bromides has also been shown to be an effective key step for the synthesis of fluorescent polyaromatic α-amino acids (Scheme 16).58 For example, the palladium acetate catalysed reaction of vinylglycinol 26 with 1-bromopyrene gave the Heck adduct in 87% yield. Hydrogenation, protecting group manipulation and oxidation gave the brightly fluorescent pyrene derived α-amino acid 27 in good overall yield. Tripeptide 28 formed from α-amino acid 27 was shown to bind to the viral RNA trans-activation response element with a Kd value of 50 nM and exhibited antibacterial activity against Bacillus subtilis.
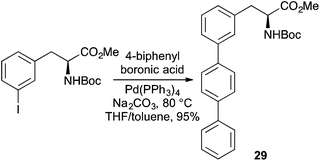
The most common coupling reaction used for the synthesis of fluorescent polyaromatic α-amino acids is the Suzuki–Miyaura reaction of α-amino acids bearing vinyl or aryl halide side-chains with polyaromatic boronic acids.59–63 This has resulted in the preparation of a range of novel pyrene or terphenyl derived α-amino acids that have been incorporated into proteins and used as fluorescent tags or in FRET experiments.61,63 For example, Hecht and co-workers used a Suzuki–Miyaura reaction of iodinated phenylalanine derivatives with biphenylboronic acids to give regioisomeric terphenyl analogues such as 29 (Scheme 17).60 These compounds were used to activate suppressor tRNA transcripts and incorporated into sterically accessible positions of DHFR from E. coli, with minimal disruption to structure or function. The potential of these compounds to act as a FRET pair with a coumarin derived α-amino acid within the mutant reductase was also demonstrated.
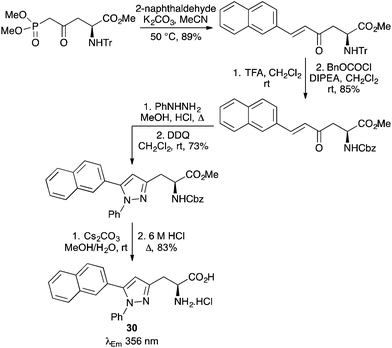
Fluorescent polyaromatic α-amino acids have also been prepared using HWE reactions of phosphonate ester derived α-amino acids with various polyaromatic aldehydes.64,65 Garbay and co-workers used such a process to prepare naphthyl-derived dehydroalanine derivatives, which were then subjected to an asymmetric hydrogenation reaction to give the L-amino acids.64 Many of these compounds displayed higher quantum yields and emission maximum wavelengths than tryptophan or tyrosine. A phosphonate ester derived L-aspartic acid derivative has been used in a HWE reaction for the synthesis of α-amino acids bearing aromatic and polyaromatic enone side-chains (Scheme 18).65 The enones were found to be excellent substrates for a highly regioselective condensation/aza-Michael reaction with phenylhydrazine. The resulting pyrazolines were oxidised with DDQ and following deprotection, gave a range of aromatic and polyaromatic pyrazole containing α-amino acids. Compounds such as naphthyl analogue 30 showed intense fluorescence at an emission maximum of 356 nm.
7. Conclusions
With a better understanding of how molecular structure affects the intensity and wavelength of fluorescent characteristics,66 significant progress has been made in designing unnatural α-amino acids for specific applications in medicine and biology. The continued development of new synthetic methodology as well as application and optimisation of well-established transition metal catalysed coupling technology has allowed the fine-tuning of the fluorescent properties of a diverse range of next generation unnatural α-amino acids. The combination of these developments with the advances in chemical biology methods for site-specific incorporation of these dyes into proteins has allowed the unparalleled investigation of a range of fundamental biological processes at the molecular level. In particular, the use of small unnatural α-amino acids that mimic tyrosine or tryptophan has resulted in labelled active proteins with minimal disruption to structure and local conformation. These constructs in combination with processes such as FRET experiments67 have led to significant insight into a wide range of enzyme mechanisms and cellular processes. Despite these advances, challenges still remain. Research is underway to further refine the structure and photophysical properties of fluorescent unnatural α-amino acids for application in a wider range of environment-sensitive processes and this includes producing compounds with higher quantum yields with fluorescence at longer wavelengths for new applications in medical research.68Acknowledgements
Financial support from the University of Glasgow and the EPSRC (studentship to AHH, EP/K503058) is gratefully acknowledged.Notes and references
- (a) B. Valeur and M. N. Berberan-Santos, Molecular Fluorescence: Principles and Applications, Wiley-VCH, Weinheim, 2012 Search PubMed; (b) W. T. Mason, Fluorescent and Luminescent Probes for Biological Activity—A practical guide to technology for quantitative real-time analysis, Academic Press, San Diego, 1999 Search PubMed.
- For some recent reviews, see: (a) K. D. Dorfman, S. B. King, D. W. Olson, J. D. P. Thomas and D. R. Tree, Chem. Rev., 2013, 113, 2584 CrossRef CAS PubMed; (b) S. Pitchiaya, L. A. Heinicke, T. C. Custer and N. G. Walter, Chem. Rev., 2014, 114, 3224 CrossRef CAS PubMed; (c) C. J. Chang, T. Gunnlaugsson and T. D. James, Chem. Soc. Rev., 2015, 44, 4176 RSC and articles within this issue; (d) L.-Y. Niu, Y.-Z. Chen, H.-R. Zheng, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Chem. Soc. Rev., 2015, 44, 6143 RSC; (e) A. B. Chinen, C. M. Guan, J. R. Ferrer, S. N. Barnaby, T. J. Merkel and C. A. Mirkin, Chem. Rev., 2015, 115, 10530 CrossRef CAS PubMed; (f) A. Fernández and M. Vendrell, Chem. Soc. Rev., 2016, 45, 1182 RSC; (g) X. Chen, F. Wang, J. Y. Hyun, T. Wei, J. Qiang, X. Ren, I. Shin and J. Yoon, Chem. Soc. Rev., 2016, 45, 2976 RSC.
- R. Y. Tsien, Annu. Rev. Biochem., 1998, 67, 509 CrossRef CAS PubMed.
- R. W. Sinkeldam, N. J. Greco and Y. Tor, Chem. Rev., 2010, 110, 2579 CrossRef CAS PubMed and references therein.
- M. S. T. Gonçalves, Chem. Rev., 2009, 109, 190 CrossRef PubMed.
- A. R. Katritzky and T. Narindoshvili, Org. Biomol. Chem., 2009, 7, 627 CAS.
- For the synthesis and application of fluorescent peptides, see: (a) S. B. VanEngelenburg and A. E. Palmer, Curr. Opin. Chem. Biol., 2008, 12, 60 CrossRef CAS PubMed; (b) T. Terai and T. Nagano, Curr. Opin. Chem. Biol., 2008, 12, 515 CrossRef CAS PubMed; (c) E. Pazos, O. Vázquez, J. L. Mascareñas and M. E. Vázquez, Chem. Soc. Rev., 2009, 38, 3348 RSC; (d) R. Kubota and I. Hamachi, Chem. Soc. Rev., 2015, 44, 4454 RSC; (e) T. Kowada, H. Maeda and K. Kikuchi, Chem. Soc. Rev., 2015, 44, 4953 RSC.
- A. T. Krueger and B. Imperiali, ChemBioChem, 2013, 14, 788 CrossRef CAS PubMed.
- L. C. Speight, M. Samanta and E. J. Petersson, Aust. J. Chem., 2014, 67, 686 CrossRef CAS.
- For general methods for the asymmetric synthesis of α-amino acids, see: (a) R. M. Williams, Synthesis of Optically Active α-Amino Acids, Pergamon, Oxford, 1989 Search PubMed; (b) R. O. Duthaler, Tetrahedron, 1994, 50, 1539 CrossRef CAS; (c) N. M. Kelly, A. Sutherland and C. L. Willis, Nat. Prod. Rep., 1997, 14, 205 RSC; (d) C. Cativiela and M. D. Diaz-de-Villegas, Tetrahedron: Asymmetry, 1998, 9, 3517 CrossRef CAS; (e) C. Cativiela and M. D. Diaz-de-Villegas, Tetrahedron: Asymmetry, 2000, 11, 645 CrossRef CAS; (f) C. Cativiela and M. D. Diaz-de-Villegas, Tetrahedron: Asymmetry, 2007, 18, 569 CrossRef CAS; (g) C. Cativiela and M. Ordóñez, Tetrahedron: Asymmetry, 2009, 20, 1 CrossRef CAS PubMed; (h) Amino Acids, Peptides and Proteins in Organic Chemistry, ed. A. B. Hughes, Wiley-VCH, Weinheim, 2009, vol. 1 Search PubMed; (i) V. A. Soloshonok, H. Ueki and T. K. Ellis, Synlett, 2009, 704 CrossRef CAS; (j) R. Smits, C. D. Cadicamo, K. Burger and B. Koksch, Chem. Soc. Rev., 2008, 37, 1727 RSC; (k) J. Michaux, G. Niel and J.-M. Campagne, Chem. Soc. Rev., 2009, 38, 2093 RSC.
- J. M. Goldberg, L. C. Speight, M. W. Fegley and E. J. Petersson, J. Am. Chem. Soc., 2012, 134, 6088 CrossRef CAS PubMed.
- S. Chen, L. Wang, N. E. Fahmi, S. J. Benkovic and S. M. Hecht, J. Am. Chem. Soc., 2012, 134, 18883 CrossRef CAS PubMed.
- H. S. Lee, J. Guo, E. A. Lemke, R. D. Dimla and P. G. Schultz, J. Am. Chem. Soc., 2009, 131, 12921 CrossRef CAS PubMed.
- (a) E. Kuru, H. V. Hughes, P. J. Brown, E. Hall, S. Tekkam, F. Cava, M. A. de Pedro, Y. V. Brun and M. S. VanNieuwenhze, Angew. Chem., Int. Ed., 2012, 51, 12519 CrossRef CAS PubMed; (b) E. Kuru, S. Tekkam, E. Hall, Y. V. Brun and M. S. VanNieuwenhze, Nat. Protoc., 2015, 10, 33 CrossRef CAS PubMed.
- J. M. Beechem and L. Brand, Ann. Rev. Biochem., 1985, 54, 43 CrossRef CAS PubMed.
- M. De Zotti, S. Bobone, A. Bortolotti, E. Longo, B. Biondi, C. Peggion, F. Formaggio, C. Toniolo, A. D. Bona, B. Kaptein and L. Stella, Chem. Biodiversity, 2015, 12, 513 CAS.
- G. Pereira, H. Vilaça and P. M. T. Ferreira, Amino Acids, 2013, 44, 335 CrossRef CAS PubMed.
- X. Wang, J. Hu, G. Zhang and S. Liu, J. Am. Chem. Soc., 2014, 136, 9890 CrossRef CAS PubMed.
- P. Cheruku, J.-H. Huang, H.-J. Yen, R. S. Iyer, K. D. Rector, J. S. Martinez and H.-L. Wang, Chem. Sci., 2015, 6, 1150 RSC.
- J. Wen, L.-L. Zhu, Q.-W. Bi, Z.-Q. Shen, X.-X. Li, X. Li, Z. Wang and Z. Chen, Chem. – Eur. J., 2014, 20, 974 CrossRef CAS PubMed.
- T. J. Williams, A. J. Reay, A. C. Whitwood and I. J. S. Fairlamb, Chem. Commun., 2014, 50, 3052 RSC.
- (a) P. Talukder, S. Chen, P. M. Arce and S. M. Hecht, Org. Lett., 2014, 16, 556 CrossRef CAS PubMed; (b) P. Talukder, S. Chen, C. T. Liu, E. A. Baldwin, S. J. Benkovic and S. M. Hecht, Bioorg. Med. Chem., 2014, 22, 5924 CrossRef CAS PubMed; (c) P. Talukder, S. Chen, B. Roy, P. Yakovchuk, M. M. Spiering, M. P. Alam, M. M. Madathil, C. Bhattacharya, S. J. Benkovic and S. M. Hecht, Biochemistry, 2015, 54, 7457 CrossRef CAS PubMed.
- (a) C. I. C. Esteves, A. M. F. Silva, M. M. M. Raposo and S. P. G. Costa, Tetrahedron, 2009, 65, 9373 CrossRef CAS; (b) C. I. C. Esteves, M. M. M. Raposo and S. P. G. Costa, Tetrahedron, 2010, 66, 7479 CrossRef CAS; (c) C. I. C. Esteves, M. M. M. Raposo and S. P. G. Costa, Amino Acids, 2011, 40, 1065 CrossRef CAS PubMed.
- (a) A. R. Katritzky, T. Narindoshvili and P. Angrish, Synthesis, 2008, 2013 CrossRef CAS; (b) A. R. Katritzky, S. Ozcan and E. Todadze, Org. Biomol. Chem., 2010, 8, 1296 RSC; (c) A. V. Ivanov, I. A. Ushakov, K. B. Pertushenko, A. I. Mikhaleva and B. A. Trofimov, Eur. J. Org. Chem., 2010, 4554 CrossRef CAS; (d) M. D. Mertens and M. Gütschow, Synthesis, 2014, 2191 CAS; (e) D. Häuβler and M. Gütschow, Synthesis, 2016, 245 Search PubMed.
- (a) L. K. Ottesen, J. W. Jaroszewski and H. Franzyk, J. Org. Chem., 2010, 75, 4983 CrossRef CAS PubMed; (b) L. Wirtz, D. Auerbach, G. Jung and U. Kazmaier, Synthesis, 2012, 2005 CAS.
- (a) M. Tasior, D. Kim, S. Singha, M. Krzeszewski, K. H. Ahn and D. T. Gryko, J. Mater. Chem. C, 2015, 3, 1421 RSC; (b) F. G. Medina, J. G. Marrero, M. Macías-Alonso, M. C. González, I. Córdova-Guerrero, A. G. T. García and S. Osegueda-Robles, Nat. Prod. Rep., 2015, 32, 1472 RSC; (c) K. P. Barot, S. V. Jain, L. Kremer, S. Singh and M. D. Ghate, Med. Chem. Res., 2015, 24, 2771 CrossRef CAS.
- (a) F. A. Bennett, D. J. Barlow, A. N. O. Dodoo, R. C. Hider, A. B. Lansley, M. J. Lawrence, C. Marriott and S. S. Bansal, Tetrahedron Lett., 1997, 38, 7449 CrossRef CAS; (b) C. G. Knight, Lett. Pept. Sci., 1998, 5, 1 CAS; (c) P. Kele, G. Sui, Q. Huo and R. M. Leblanc, Tetrahedron: Asymmetry, 2000, 11, 4959 CrossRef CAS; (d) G. Sui, P. Kele, J. Orbulescu, Q. Huo and R. M. Leblanc, Lett. Pept. Sci., 2002, 8, 47 Search PubMed; (e) W. Wang and H. Li, Tetrahedron Lett., 2004, 45, 8479 CrossRef CAS.
- M.-P. Brun, L. Bischoff and C. Garbay, Angew. Chem., Int. Ed., 2004, 43, 3432 CrossRef CAS PubMed.
- J. Ge, L. Li and S. Q. Yao, Chem. Commun., 2011, 47, 10939 RSC.
- T. Koopmans, M. van Haren, L. Q. van Ufford, J. M. Beekman and N. I. Martin, Bioorg. Med. Chem., 2013, 21, 553 CrossRef CAS PubMed.
- D. Häuβler and M. Gütschow, Heteroatom Chem., 2015, 26, 367 CrossRef.
- M. Braun and T. Dittrich, Beilstein J. Org. Chem., 2010, 6, 69 Search PubMed.
- X. Xu, X. Hu and J. Wang, Beilstein J. Org. Chem., 2013, 9, 254 CrossRef CAS PubMed.
- L. Schmidt, T. Doroshenko, P. Barbie, A. Grüter, G. Jung and U. Kazmaier, Synthesis, 2016, 48, 3077 CrossRef CAS.
- M. Albrecht, A. Lippach, M. P. Exner, J. Jerbi, M. Springborg, N. Budisa and G. Wenz, Org. Biomol. Chem., 2015, 13, 6728 CAS.
- K. Guzow, K. Kornowska and W. Wiczk, Tetrahedron Lett., 2009, 50, 2908 CrossRef CAS.
- (a) C. Li, J. Tang and J. Xie, Tetrahedron, 2009, 65, 7935 CrossRef CAS; (b) C. Li, E. Henry, N. K. Mani, J. Tang, J.-C. Brochon, E. Deprez and J. Xie, Eur. J. Org. Chem., 2010, 2395 CrossRef CAS; (c) Y.-B. Ruan, Y. Yu, C. Li, N. Bogliotti, J. Tang and J. Xie, Tetrahedron, 2013, 69, 4603 CrossRef CAS.
- S. Sasaki, H. Suzuki, H. Ouchi, T. Asakawa, M. Inai, R. Sakai, K. Shimamoto, Y. Hamashima and T. Kan, Org. Lett., 2014, 16, 564 CrossRef CAS PubMed.
- C. Hoppmann, U. Alexiev, E. Irran and K. Rück-Braun, Tetrahedron Lett., 2013, 54, 4585 CrossRef CAS.
- L. C. Speight, A. K. Muthusamy, J. M. Goldberg, J. B. Warner, R. F. Wissner, T. S. Willi, B. F. Woodman, R. A. Mehl and E. J. Petersson, J. Am. Chem. Soc., 2013, 135, 18806 CrossRef CAS PubMed.
- J. P. Wolfe, S. Wagaw, J. F. Marcoux and S. L. Buchwald, Acc. Chem. Res., 1998, 31, 805 CrossRef CAS.
- (a) A. V. Strizhak, V. Y. Postupalenko, V. V. Shvadchak, N. Morellet, E. Guittet, V. G. Pivovarenko, A. S. Klymchenko and Y. Mély, Bioconjugate Chem., 2012, 23, 2434 CrossRef CAS PubMed; (b) V. Y. Postupalenko, O. M. Zamotaiev, V. V. Shvadchak, A. V. Strizhak, V. G. Pivovarenko, A. S. Klymchenko and Y. Mély, Bioconjugate Chem., 2013, 24, 1998 CrossRef CAS PubMed.
- T. R. Gormley and W. I. O'Sullivan, Tetrahedron, 1973, 29, 369 CrossRef CAS.
- M. Nitz, A. R. Mezo, M. H. Ali and B. Imperiali, Chem. Commun., 2002, 1912 RSC.
- B. E. Cohen, T. B. McAnaney, E. S. Park, Y. N. Jan, S. G. Boxer and L. Y. Jan, Science, 2002, 296, 1700 CrossRef CAS PubMed.
- M. E. Vázquez, D. M. Rothman and B. Imperiali, Org. Biomol. Chem., 2004, 2, 1965 Search PubMed.
- G. Loving and B. Imperiali, J. Am. Chem. Soc., 2008, 130, 13630 CrossRef CAS PubMed.
- J. Maity, D. Honcharenko and R. Strömberg, Tetrahedron Lett., 2015, 56, 4780 CrossRef CAS.
- A. Chatterjee, J. Guo, H. S. Lee and P. G. Schultz, J. Am. Chem. Soc., 2013, 135, 12540 CrossRef CAS PubMed.
- M. Wen, X. Guo, P. Sun, L. Xiao, J. Li, Y. Xiong, J. Bao, T. Xue, L. Zhang and C. Tian, Chem. Commun., 2015, 51, 8153 RSC.
- Z. Xiang and L. Wang, J. Org. Chem., 2011, 76, 6367 CrossRef CAS PubMed.
- E. Benedetti, A. B. E. Veliz, M. Charpenay, L. S. Kocsis and K. M. Brummond, Org. Lett., 2013, 15, 2578 CrossRef CAS PubMed.
- L. S. Fowler, D. Ellis and A. Sutherland, Org. Biomol. Chem., 2009, 7, 4309 CAS.
- A. R. Mohite and R. G. Bhat, J. Org. Chem., 2012, 77, 5423 CrossRef CAS PubMed.
- P. M. T. Ferreira, L. S. Monteiro, G. Pereira, E. M. S. Castanheira and C. G. Frost, Eur. J. Org. Chem., 2013, 550 CrossRef CAS.
- G. Pereira, E. M. S. Castanheira, P. Schellenberg, M. Belsley, P. M. T. Ferreira and L. S. Monteiro, Tetrahedron, 2013, 69, 10254 CrossRef CAS.
- S. Kotha and M. Meshram, Synthesis, 2014, 1525 CrossRef.
- M. Suhartono, M. Weidlich, T. Stein, M. Karas, G. Dürner and M. W. Göbel, Eur. J. Org. Chem., 2008, 1608 CrossRef CAS.
- A. S. Abreu, E. M. S. Castanheira, P. M. T. Ferreira, L. S. Monteiro, G. Pereira and M.-J. R. P. Queiroz, Eur. J. Org. Chem., 2008, 5697 CrossRef CAS.
- S. Chen, N. E. Fahmi, C. Bhattacharya, L. Wang, Y. Jin, S. J. Benkovic and S. M. Hecht, Biochemistry, 2013, 52, 8580 CrossRef CAS PubMed.
- S. Chen, N. E. Fahmi, L. Wang, C. Bhattacharya, S. J. Benkovic and S. M. Hecht, J. Am. Chem. Soc., 2013, 135, 12924 CrossRef CAS PubMed.
- M. Dobmeier, P. Maity and B. König, Helv. Chim. Acta, 2014, 97, 1061 CrossRef CAS.
- J. S. Lampkowski, D. M. Uthappa and D. D. Young, Bioorg. Med. Chem. Lett., 2015, 25, 5277 CrossRef CAS PubMed.
- H. Chen, J.-P. Luzy, N. Gresh and C. Garbay, Eur. J. Org. Chem., 2006, 2329 CrossRef CAS.
- L. Gilfillan, R. Artschwager, A. H. Harkiss, R. M. J. Liskamp and A. Sutherland, Org. Biomol. Chem., 2015, 13, 4514 CAS.
- (a) L. D. Lavis and R. T. Raines, ACS Chem. Biol., 2008, 3, 142 CrossRef CAS PubMed; (b) L. D. Lavis and R. T. Raines, ACS Chem. Biol., 2014, 9, 855 CrossRef CAS PubMed.
- K. Kikuchi, Chem. Soc. Rev., 2010, 39, 2048 RSC.
- V. J. Pansare, S. Hejazi, W. J. Faenza and R. K. Prud'homme, Chem. Mater., 2012, 24, 812 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |