Abstract
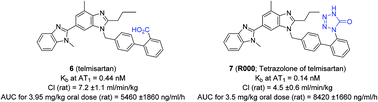
Matched molecular pair analysis was used to evaluate the ability of a tetrazolone group to act as a bioisostere of a carboxylic acid. Compound 7, a tetrazolone of the anti-hypertensive drug, telmisartan 6, was shown to be a potent AT1 antagonist (Kb = 0.14 nM), with activity comparable to telmisartan itself (Kb = 0.44 nM). Additionally, compound 9, a tetrazolone congener of the marketed anti-cancer agent, bexarotene 8, was shown to be an agonist at the retinoid X receptor alpha (EC50 = 64 nM). Compounds containing a tetrazolone group showed similar microsomal stability and plasma protein binding to marketed acid counterparts, while also reducing the value for clog P. Furthermore, compound 7 displayed an improved rat pharmacokinetic profile cf. telmisartan 6. Taken together, the results demonstrate that a tetrazolone group may serve as a bioisostere for a carboxylic acid.
- This article is part of the themed collection: Contemporary Synthetic Chemistry in Drug Discovery

 Please wait while we load your content...
Please wait while we load your content...