Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A step-economical multicomponent synthesis of 3D-shaped aza-diketopiperazines and their drug-like chemical space analysis†
Pierre
Regenass
,
Stéphanie
Riché
,
Florent
Péron
,
Didier
Rognan
,
Marcel
Hibert
,
Nicolas
Girard
* and
Dominique
Bonnet
 *
*
Laboratoire d'Innovation Thérapeutique, UMR7200 CNRS/Université de Strasbourg, Labex MEDALIS, Faculté de Pharmacie, 74 route du Rhin, 67412 Illkirch, France. E-mail: nicolas.girard@unistra.fr; dominique.bonnet@unistra.fr
First published on 22nd August 2016
Abstract
A rapid and atom economical multicomponent synthesis of complex aza-diketopiperazines (aza-DKPs) driven by Rh(I)-catalyzed hydroformylation of alkenylsemicarbazides is described. Combined with catalytic amounts of acid and the presence of nucleophilic species, this unprecedented multicomponent reaction (MCR) enabled the formation of six bonds and a controlled stereocenter from simple substrates. The efficacy of the strategy was demonstrated with a series of various allyl-substituted semicarbazides and nucleophiles leading to the preparation of 3D-shaped bicyclic aza-DKPs. Moreover, an analysis of their 3D molecular descriptors and “drug-likeness” properties highlights not only their originality in the chemical space of aza-heterocycles but also their great potential for medicinal chemistry.
Introduction
Low molecular-weight organic molecules are a major source of pharmacological probes and/or drug candidates.1 Recent achievements in Pd-catalyzed C(sp2)–C(sp2) coupling reactions have greatly facilitated the syntheses of small-size compounds.2,3 While these advances have largely contributed to drug discovery, mainly compounds with greater unsaturation, aromaticity and flatness were obtained.4 But, as recently reported, such compounds are less likely to succeed in clinical trials than saturated ones.5 Indeed, increasing saturation in molecules results in more complex three dimensional 3D-shaped structures with greater potential to better complement the spatial subtleties of target proteins, thus increasing their selectivity and reducing off-target liabilities. In addition, saturation also greatly improves aqueous solubility and as a consequence ameliorates pharmacokinetic properties while decreasing potential toxicity. Finally, it has been observed that the number of stereogenic centers increases from early hit discovery to late drug candidate stages, highlighting the importance of molecular complexity in drug development.4In this context, we embarked on a general programme aiming to design efficient and facile routes to complex 3D-shaped molecules containing sp3 hybridized carbons and stereocenters with potential application in medicinal chemistry. Indeed, we described recently the access to novel aza-diketopiperazine (aza-DKP) scaffolds, which represent the smallest cyclo aza-peptides described to date (Fig. 1, series A).6 Relying on a cyclohydrocarbonylation (CHC)/addition process, we also synthesized a range of bicyclic and tricyclic aza-DKPs incorporating six- or seven-membered rings appended with defined stereocenters (Fig. 1, series B and C).7 Herein, we report an unprecedented and highly efficient one-pot sequence to readily access aza-DKPs. For the first time, alkenyl-substituted semicarbazides were subjected to a Rh(I)-catalyzed hydroformylation.8 This multicomponent reaction (MCR) performed under acid catalysis and in the presence of various nucleophiles enabled the one-pot formation of six bonds and one stereocenter. Thereby, this step and atom economical approach was advantageously applied to the preparation of a range of aza-DKPs. In addition, their 3D molecular descriptors and “drug-likeness” properties were carefully evaluated to determine not only their originality in the chemical space of existing scaffolds but also their propensity to succeed in potential future clinical trials.
 | ||
| Fig. 1 Chemical structures of recently synthesized aza-DKP scaffolds.6,7 | ||
Results and discussion
While DKPs represent an important heterocyclic core present in synthetic and natural products, their nitrogen analogues, aza-DKPs, are less well-known and provide interesting opportunities for lead discovery. To access this novel class of underprivileged scaffold, very few practical synthetic routes are available.9,10 For bicyclic or tricyclic aza-DKPs B and C (Fig. 1), the only synthetic approach reported to date involves a four-step process starting from alkyl-amino esters.6 Thereby, to facilitate the access to diverse aza-DKPs 4, we decided to develop a rapid and convenient approach that relies on an unprecedented MCR encompassing five transformations from semicarbazides 1 (Scheme 1).A novel multicomponent reaction
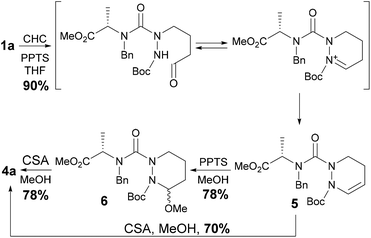
Firstly, to develop this novel approach, we needed to evaluate the fate of semicarbazides 1 upon hydroformylation (Scheme 2). Thus, semicarbazide model 1a, readily obtained from N-alkyl amino ester,6 was subjected to hydroformylation in the presence of Rh(CO)2(acac)/BiPhePhos,11,12 CO/H2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5 bar) and pyridinium p-toluenesulfonate (PPTS). Under these experimental conditions, 1a was converted into tetrahydropyridazine 5 in excellent yield (90%). This result not only shows that the hydroformylation is compatible with semicarbazides of type 1 but also shows that the Boc protected secondary amine is nucleophilic enough to spontaneously promote the cyclization with the aldehyde moiety to form the iminium intermediate. Interestingly, the resulting original unsaturated terahydropyridazine 5 can be viewed as a precursor of topographically constrained α-aza-amino acids.13 Then, the nucleophilic addition of MeOH to 5 in the presence of PPTS (0.5 equiv.) gave access to 6 in good yield (78%) but a low dr (60
1, 5 bar) and pyridinium p-toluenesulfonate (PPTS). Under these experimental conditions, 1a was converted into tetrahydropyridazine 5 in excellent yield (90%). This result not only shows that the hydroformylation is compatible with semicarbazides of type 1 but also shows that the Boc protected secondary amine is nucleophilic enough to spontaneously promote the cyclization with the aldehyde moiety to form the iminium intermediate. Interestingly, the resulting original unsaturated terahydropyridazine 5 can be viewed as a precursor of topographically constrained α-aza-amino acids.13 Then, the nucleophilic addition of MeOH to 5 in the presence of PPTS (0.5 equiv.) gave access to 6 in good yield (78%) but a low dr (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40). The treatment of the latter in the presence of a strong acid (CSA, 0.5 equiv.) in MeOH at 70 °C initiated a second cyclization towards 4a, which was obtained in good yield (78%) and, more surprisingly, a much greater diastereoselectivity than that obtained for 6 (93
40). The treatment of the latter in the presence of a strong acid (CSA, 0.5 equiv.) in MeOH at 70 °C initiated a second cyclization towards 4a, which was obtained in good yield (78%) and, more surprisingly, a much greater diastereoselectivity than that obtained for 6 (93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 vs. 60
7 vs. 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, respectively). This result could be ascribed to the reversibility of the hemiaminal formation under strongly acidic conditions likely evolving towards the thermodynamically more stable trans-isomer. To support this result, the energy differences (ΔH based on MM2 calculations) between the cis and the trans isomers for 6 and 4a were evaluated. Minimized structures for each diastereoisomer are depicted in the ESI.† Thereby, the energy difference for 6 is 1.39 kcal mol−1, resulting in the formation of both isomers with a lower dr than for 4a that displays a higher ΔH (2.01 kcal mol−1) in favor of the trans isomer. In search of a method for reducing the number of steps, we found that tetrahydropyridazine 5 could also be advantageously treated with CSA (0.5 equiv.) in MeOH to directly lead to 4a in good yield (70%) and high diastereoselectivity (93
40, respectively). This result could be ascribed to the reversibility of the hemiaminal formation under strongly acidic conditions likely evolving towards the thermodynamically more stable trans-isomer. To support this result, the energy differences (ΔH based on MM2 calculations) between the cis and the trans isomers for 6 and 4a were evaluated. Minimized structures for each diastereoisomer are depicted in the ESI.† Thereby, the energy difference for 6 is 1.39 kcal mol−1, resulting in the formation of both isomers with a lower dr than for 4a that displays a higher ΔH (2.01 kcal mol−1) in favor of the trans isomer. In search of a method for reducing the number of steps, we found that tetrahydropyridazine 5 could also be advantageously treated with CSA (0.5 equiv.) in MeOH to directly lead to 4a in good yield (70%) and high diastereoselectivity (93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
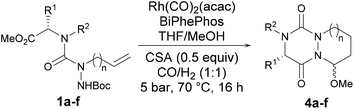
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). Thus, bicyclic aza-DKP 4a was obtained from semicarbazide 1a in a two-step sequence in 63% overall yield. After validating the hydroformylation on allyl-substituted semicarbazide, we focused our attention on the development of a multicomponent reaction to straightforwardly convert 1a into 4a. The optimized experimental conditions determined above were advantageously combined. Thereby, 1a was treated in the presence of CSA and the Rh/BiPhePhos complex under catalytic conditions in MeOH/THF at 70 °C under 5 bar of CO/H2 (Table 1, entry 1). In our first attempt, we were pleased to obtain the expected bicyclic aza-DKP 4a. It is noteworthy that the concentration of CSA in MeOH/THF was found to be crucial for driving the one-pot sequence to completion. After optimization, 4a was obtained in good yield (69%) and the same dr (93
7). Thus, bicyclic aza-DKP 4a was obtained from semicarbazide 1a in a two-step sequence in 63% overall yield. After validating the hydroformylation on allyl-substituted semicarbazide, we focused our attention on the development of a multicomponent reaction to straightforwardly convert 1a into 4a. The optimized experimental conditions determined above were advantageously combined. Thereby, 1a was treated in the presence of CSA and the Rh/BiPhePhos complex under catalytic conditions in MeOH/THF at 70 °C under 5 bar of CO/H2 (Table 1, entry 1). In our first attempt, we were pleased to obtain the expected bicyclic aza-DKP 4a. It is noteworthy that the concentration of CSA in MeOH/THF was found to be crucial for driving the one-pot sequence to completion. After optimization, 4a was obtained in good yield (69%) and the same dr (93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) as that described above.
7) as that described above.
| Entry | Amino acid | R1 | R2 | n | Yielda (%) | dr (trans/cis)b |
|---|---|---|---|---|---|---|
| a Isolated yields. b Diastereomeric ratios were determined by 1H NMR or HPLC analysis of the crude reaction mixtures. c 1.5 equiv. of CSA were used instead of 0.5 equiv. | ||||||
| 1 | L-Ala | (S)-Me | Bn | 1 | 4a (69) | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 2 | L-Ile | (S)-sec-Bu | Bn | 1 | 4b (48) | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | L-Ala | (S)-Me | iPe | 1 | 4c (32) | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 4 | L-Phe | (S)-Bn | Bn | 1 | 4d (31) | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 5 | L-Lys(Boc) | (S)-H2N(CH2)4 | Bn | 1 | 4e (25)c | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 6 | L-Ala | (S)-Me | Bn | 2 | 4f (52) | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63 63 |
Scope and limitations of the multicomponent reaction
With these promising results in hand, the scope and limitations of the MCR were then evaluated. To this end, the sequence was extended to other semicarbazides (1b–f) that were obtained in moderate to good yields (37 to 82%) from the corresponding N-alkyl amino acid esters. These substrates were then subjected to the optimized conditions mentioned above for the MCR. Isolated yields and dr for all compounds 4a–f are summarized in Table 1. In all cases, the expected bicyclic compounds were obtained in yields ranging from 25% to 69% and a high diastereoselectivity. The process was compatible with the presence of bulky substituents at R1 and R2 (Table 1, entries 2 and 3) or aromatic ones (entry 4). Functional groups such as amines (entry 5) were also tolerated and did not interfere with the Rh/BiPhePhos complex efficacy. Remarkably, the cyclization to a seven-membered ring (entry 6) was also found successful but with a lower stereoselectivity. This result can be ascribed to the energy difference between the two isomers (0.05 kcal mol−1), much below 2 kcal mol−1. Altogether, these results demonstrate that this process represents a convenient atom- and step-economical route for the preparation of complex 3D-shaped bicyclic structures starting from simple and readily accessible allyl- or homoallyl-substituted semicarbazides.Extension to other nucleophiles
To further extend the scope of the MCR, various nucleophilic species were evaluated (Table 2). To this end, semicarbazide 1a was subjected to the same one-pot sequence experimental conditions as those described above by replacing MeOH by EtOH, nPrOH, iPrOH, TMSCN or nPrSH. In the alcohol series, the efficiency of the reaction was clearly correlated to the hindered character of the nucleophile (Table 2). Indeed, whereas hemiaminal 4a (R4 = OMe, Table 1, entry 1) and 4g (R4 = OEt, entry 1) were obtained in good yields (69% and 65%, respectively), the reaction was found sluggish with more hindered R4 such as OnPr or OiPr. In this case, increasing the amount of CSA (1 equiv. vs. 0.5 equiv.) enabled us to greatly improve the efficacy of the process (Table 2, entries 2 and 3). Regardless of the nature of the alcohol, aza-DKPs were obtained with good stereoselectivities. Finally, hydroformylation performed in the presence of nPrSH or TMSCN failed to give 4j and 4k. Both nucleophiles were found to compete as a ligand for rhodium, leading to the inactivation of the Rh/BiPhePhos complex. To overcome this limitation, hydroformylation was first achieved before adding the nucleophilic species (nPrSH or TMSCN/BF3·OEt2), thus leading to the expected aza-DKPs 4j and 4k (Table 2, entries 4 and 5) in good yield (71% and 79%, respectively) but a low diastereoselectivity (73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 and 55
27 and 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45, respectively).
45, respectively).
| Entry | R4 | Yielda (%) | dr (trans/cis)b |
|---|---|---|---|
| a Isolated yields. b Diastereomeric ratios were determined by 1H NMR and/or HPLC analysis of the crude reaction mixtures. c 1 equiv. of CSA was used instead of 0.5 equiv. d Reaction performed without CSA; nPrSH or BF3·Et2O/TMSCN was added after 7 h of reaction. | |||
| 1 | OEt | 4g (65) | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 2 | OnPr | 4h (47)c | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 3 | OiPr | 4i (46)c | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 4 | SnPr | 4j (71)d | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
| 5 | CN | 4k (79)d | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
In an attempt to explain the difference of dr between the aza-DKPs, the reversibility of the nucleophilic addition was carefully investigated. Thus, the treatment of aza-DKPs 4g (R4 = OEt) and 4j (R4 = SnPr) in methanol in the presence of CSA at 70 °C led to 4a in the same isomeric ratio as those obtained during the MCR. This result tends to demonstrate that the nucleophilic addition is reversible and under thermodynamic control. The theoretical calculations of energy difference performed on both diastereoisomers of 4g–j (4g: ΔH = 2.13 kcal mol−1; 4h: ΔH = 3.83 kcal mol−1; 4i: ΔH = 4.65 kcal mol−1; 4j: ΔH = 1.80 kcal mol−1) are in agreement with the dr experimentally obtained. In contrast, aza-DKP 4k treated under the same conditions (CSA, MeOH, 70 °C) was found fully stable and did not result in the formation of 4a. In this case, the addition of CN species on the iminium intermediate is likely under kinetic control, only leading to the formation of aza-DKP 4k with a low selectivity.
3D molecular shape and “drug-likeness” properties of aza-DKPs
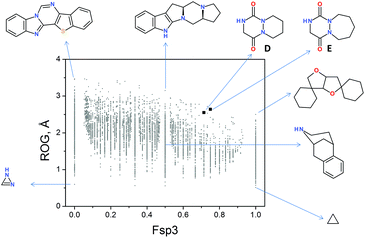
Once the novel MCR was developed, we wanted to evaluate the “drug-likeness” properties of the two model aza-DKP scaffolds 4a and 4f, incorporating six- and seven-membered rings, respectively. As previously reported, the clinical success of drug candidates is greatly influenced by their physicochemical properties, among which one of the most important is molecular shape.14 It has been shown that two dimensional (2D) molecular descriptors such as the fraction of hybridized sp3 carbon atoms (Fsp3) and the number of stereocenters correlate with clinical success.5 In addition, shape-based 3D descriptors such as the radius of gyration (ROG) and shadow indices (Shadow Xlength, SXL) explicitly describe the compactness and flatness of a compound, and therefore represent an efficient way to evaluate the propensity of a compound to advance from clinical trials to the market.15 Although predictions based solely on physicochemical properties should be taken with care, drug candidates successfully advancing to clinical trials tend to share the following properties: SXL ≤ 16.09; ROG < 4.11; Fsp3 ≥ 0.42. Additionally, the chance of a preclinical candidate passing through the different clinical trial stages was also evaluated by the quantitative estimate of the drug-likeness (QED) descriptor. Compared to the classical rule-based approaches, QED offers a more nuanced view of drug-likeness. QED values range from zero (all properties unfavorable) to one (all properties favorable).16Thereby, these descriptors were calculated for model aza-DKP trans-isomers 4a and 4f. As outlined in Table 3, both compounds meet the three abovementioned criteria (SXL, ROG and Fsp3) and display an excellent QED of 0.870 and 0.881, respectively. These results show that the novel 3D-shaped aza-DKPs gather ideal 2D and 3D molecular characteristics to succeed in the different stages of drug development, provided that these compounds efficiently bind to a target of therapeutic value and are not recognized by undesired off-targets. To further evaluate the originality of the aza-DKPs herein synthesized, we decided to compare the molecular shape of our bicyclic scaffolds incorporating six- and seven-membered rings with other heterocyclic platforms already described in the literature. To this end, the ROG values for unsubstituted aza-DKP scaffolds D and E were plotted as a function of the Fsp3 value and compared with that of 9266 unique homo and heterocyclic scaffolds derived from 5 million commercially available drug-like compounds for which a low energy 3D structure was taken as the reference (Fig. 2). Some representative scaffolds with extreme properties are also shown to illustrate the plot.
| Cpd | Shadow Xlengtha | Radius of gyrationa |
F
sp3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
QEDb |
|---|---|---|---|---|
| a ROG, SXL and Fsp3 were calculated as reported in D. C. Kombo, et al., J. Chem. Info Model., 2013, 53, 327–342. b The quantitative estimate of drug-likeness was calculated as reported in G. R. Bickerton, et al.16 | ||||
| 4a | 13.079 | 3.638 | 0.500 | 0.870 |
| 4f | 13.653 | 3.867 | 0.529 | 0.881 |
Thus, scaffolds D and E display a very high Fsp3 value, and are interestingly located in a sparsely populated area in our 2D representation of drug-like scaffold space (Fig. 2, top right hand corner). Of particular interest, scaffolds D and E complement the molecular diversity of already existing heterocyclic scaffolds (some representative ones with extreme properties) but also display optimal “drug-likeness” properties to potentially succeed in the different stages of clinical trials.
Conclusion
The preparation of highly diverse and “out-of-plane” scaffolds requires the design of efficient, rapid and facile synthetic routes. In this article, we describe for the first time the successful application of Rh(I)-catalyzed cyclohydroformylation to allyl- and homoallyl-substituted semicarbazides. Combined with catalytic amounts of acid and the presence of nucleophilic species, the unprecedented MCR enabled the formation of six bonds and a controlled stereocenter. The efficacy of the strategy was demonstrated with a series of various allyl-substituted semicarbazides and nucleophiles leading to the rapid preparation of 3D-shaped bicyclic aza-DKPs. Thus, this process represents an advance in the field of the diversity-oriented synthesis of novel aza-heterocycles. In addition, the analysis of their 3D molecular descriptors and “drug-likeness” properties highlighted not only their originality in the chemical space of aza-heterocycles but also their high propensity to succeed in potential future clinical trials.Acknowledgements
This work has been published in the frame of the LABEX ANR-10-LABX-0034_Medalis and received financial support from the French government managed by the Agence Nationale de la Recherche under Programme d'investissement d'avenir. This work was also supported by the Centre National de la Recherche Scientifique (CNRS), the Université de Strasbourg (Unistra) and the French Ministère de l'Education Nationale, de l'Enseignement Supérieur et de la Recherche (Pierre Regenass fellowship). Dr André Mann is kindly acknowledged for his comments on the manuscript. We are grateful to Barbara Schaeffer and Justine Viéville for NMR experiments and Patrick Wehrung for MS analyses (Service Commun d'Analyse, Unistra).Notes and references
- B. R. Stockwell, Nature, 2004, 432, 846–854 CrossRef CAS PubMed.
- S. D. Walker, T. E. Barder, J. R. Martinelli and S. L. Buchwald, Angew. Chem., Int. Ed., 2004, 43, 1871–1876 CrossRef CAS PubMed.
- D. B. Zhao, J. S. You and C. W. Hu, Chem. – Eur. J., 2011, 17, 5466–5492 CrossRef CAS PubMed.
- W. P. Walters, J. Green, J. R. Weiss and M. A. Murcko, J. Med. Chem., 2011, 54, 6405–6416 CrossRef CAS PubMed.
- F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752–6756 CrossRef CAS PubMed.
- D. Bonnet, J. F. Margathe, S. Radford, E. Pflimlin, S. Riche, P. Doman, M. Hibert and A. Ganesan, ACS Comb. Sci., 2012, 14, 323–334 CrossRef CAS PubMed.
- P. Regenass, J. F. Margathe, A. Mann, J. Suffert, M. Hibert, N. Girard and D. Bonnet, Chem. Commun., 2014, 50, 9657–9660 RSC.
- I. Ojima, M. Tzamarioudaki and D. Bonnafoux, The Hydroformylation Reaction, Wiley, New York, 2000 Search PubMed.
- R. A. Ivanovich, J. F. Vincent-Rocan, E. B. Elkaeed and A. M. Beauchemin, Org. Lett., 2015, 17, 4898–4901 CrossRef CAS PubMed.
- C. B. Bourguet, C. Proulx, S. Klocek, D. Sabatino and W. D. Lubell, J. Pept. Sci., 2010, 16, 284–296 CrossRef CAS PubMed.
- B. Breit and W. Seiche, Synthesis, 2001, 1–36 CrossRef CAS.
- G. D. Cuny and S. L. Buchwald, J. Am. Chem. Soc., 1993, 115, 2066–2068 CrossRef CAS.
- I. Torrini, G. P. Zecchini, M. P. Paradisi, G. Mastropietro, G. Lucente, E. Gavuzzo and F. Mazza, Tetrahedron, 1999, 55, 2077–2090 CrossRef CAS.
- A. Nicholls, G. B. McGaughey, R. P. Sheridan, A. C. Good, G. Warren, M. Mathieu, S. W. Muchmore, S. P. Brown, J. A. Grant, J. A. Haigh, N. Nevins, A. N. Jain and B. Kelley, J. Med. Chem., 2010, 53, 3862–3886 CrossRef CAS PubMed.
- D. C. Kombo, K. Tallapragada, R. Jain, J. Chewning, A. A. Mazurov, J. D. Speake, T. A. Hauser and S. Toler, J. Chem. Inf. Model., 2013, 53, 327–342 CrossRef CAS PubMed.
- G. R. Bickerton, G. V. Paolini, J. Besnard, S. Muresan and A. L. Hopkins, Nat. Chem., 2012, 4, 90–98 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and analytical data for all the compounds. See DOI: 10.1039/c6ob01434h |
| This journal is © The Royal Society of Chemistry 2016 |