Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Design, synthesis and biological evaluation of 6-aryl-1,6-dihydro-1,3,5-triazine-2,4-diamines as antiplasmodial antifolates†
Anna C. U.
Lourens
ab,
David
Gravestock
ac,
Robyn L.
van Zyl
d,
Heinrich C.
Hoppe
ae,
Natasha
Kolesnikova
a,
Supannee
Taweechai
f,
Yongyuth
Yuthavong
f,
Sumalee
Kamchonwongpaisan
f and
Amanda L.
Rousseau
*ag
aCSIR Biosciences, Meiring Naude Road, Pretoria, 0001 Gauteng, South Africa. E-mail: Amanda.Rousseau@wits.ac.za
bPharmaceutical Chemistry, School of Pharmacy, North-West University, Private Bag X6001, Potchefstroom, 2520, South Africa
cSyngenta, Jealott's Hill International Research Centre, Bracknell, Berkshire RG42 6EY, UK
dPharmacology Division, Department of Pharmacy and Pharmacology, WITS Research Institute for Malaria (WRIM), Faculty of Health Sciences, University of the Witwatersrand, 7 York Road, Parktown 2193, South Africa
eDepartment of Biochemistry and Microbiology, Rhodes University, Grahamstown 6140, South Africa
fBIOTEC, National Science and Technology Development Agency, Thailand Science Park, Pathumthani 12120, Thailand
gMolecular Sciences Institute, School of Chemistry, University of the Witwatersrand, Private Bag 3, PO WITS, 2050, South Africa
First published on 25th July 2016
Abstract
The design, synthesis and biological evaluation of a series of 6-aryl-1,6-dihydro-1,3,5-triazine-2,4-diamines is described. These compounds exhibited in vitro antiplasmodial activity in the low nanomolar range against both drug sensitive and drug resistant strains of P. falciparum, with 1-(3-(2,4-dichlorophenoxy)propyl)-6-phenyl-1,6-dihydro-1,3,5-triazine-2,4-diamine hydrochloride identified as the most potent compound from this series against the drug resistant FCR-3 strain (IC50 2.66 nM). The compounds were not toxic to mammalian cells at therapeutic concentrations and were shown to be inhibitors of parasitic DHFR in a biochemical enzyme assay.
Introduction
Malaria continues to pose a health risk to much of the world population despite a dramatic decrease in malaria morbidity over the past 15 years.1 An estimated 214 million cases of malaria and 438![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 malaria fatalities were reported in 2014, with 88% of these infections originating in Africa and caused by the parasite Plasmodium falciparum.1 The increasing occurrence of insecticide resistance and the emergence of multidrug resistant strains of the parasite threaten the progress made in the fight against malaria and highlight the need for the development of new antimalarial therapies.2
000 malaria fatalities were reported in 2014, with 88% of these infections originating in Africa and caused by the parasite Plasmodium falciparum.1 The increasing occurrence of insecticide resistance and the emergence of multidrug resistant strains of the parasite threaten the progress made in the fight against malaria and highlight the need for the development of new antimalarial therapies.2
Until the emergence of drug resistance, folate metabolism was successfully targeted for both prophylaxis and the treatment of malaria.3 Folates are essential cellular cofactors required by the parasite for a number of key processes, including the initiation of protein synthesis, and the biosynthesis of nucleotides and some amino acids.4 The malaria parasite relies on these cofactors for growth, and is able to obtain the required folate derivatives by de novo synthesis or via a folate salvage pathway.
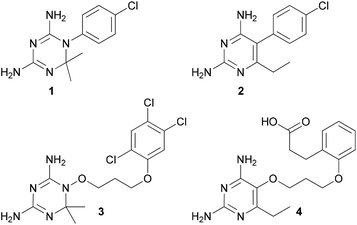
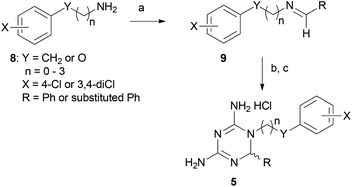
A key enzyme crucial to both pathways that has been targeted for malaria chemotherapy is dihydrofolate reductase-thymidylate synthase (DHFR-TS), a bifunctional enzyme. DHFR-TS is one of the few well-defined, validated targets in malaria chemotherapy.5 Unfortunately, point mutations in the active site of the DHFR domains of DHFR-TS have resulted in resistance to the most widely used antifolates, cycloguanil 1 and pyrimethamine 2 (Fig. 1).6 In many cases, the Ser108Asn mutation, followed by Cys59Arg, results in steric clashes with inhibitors in the DHFR active site. This has also been observed for parasitic DHFR bearing Ser108Thr and Ala16Val mutations (in the FCR-3 clone). Subsequent mutations, Asn51Ile and Ile164Leu, cause a shift in the two chains around the active site, opening it up and reducing the binding affinity of small inhibitors. By comparison, compounds containing a flexible linker between the two rings, such as WR99210 3 (Fig. 1), have been shown to maintain a high binding affinity to all mutant forms of P. falciparum DHFR.7 The flexibility imparted by this linker enables the inhibitor to avoid unfavourable contacts with mutant amino acid residues. In a similar vein, flexible pyrimethamine derivatives, including P218 4, have proven to be active against DHFR-resistant parasites.8
As part of an ongoing malarial research programme, we utilised published crystal structures of wild type and mutant P. falciparum DHFR-TS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 to design novel, flexible analogues of cycloguanil. We now report the results of this study, in which a series of compounds with in vitro antimalarial activity in the low nanomolar range were identified.10
9 to design novel, flexible analogues of cycloguanil. We now report the results of this study, in which a series of compounds with in vitro antimalarial activity in the low nanomolar range were identified.10
Results and discussion
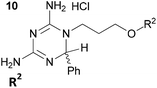
The crystal structures of both wild type PfDHFR-TS and the quadruple mutant (bearing the following mutations: Asn51Ile, Cys59Arg, Ser108Asn and Ile164Leu) were selected for in silico screening.11 In each case, the active site was defined at chain A of the DHFR region with the co-factor NADPH bound, in order to assess the comparative binding of the inhibitor molecules at the dihydrofolate binding site. The use of flexible docking protocols enabled us to identify that inhibitors bearing substituents larger than methyl at the 6-position of the dihydrotriazine ring could be accommodated in the dihydrofolate binding pocket. In particular, the side chain of Met55 is able to rotate away from the active site, enabling bulkier inhibitors to bind without disruption of the key H-bonding to Asp54, Ile14 and, in the mutant, Leu164. Examination of the literature revealed that Baker et al. had considered the use of larger substituents at the 6-position of the dihydrotriazine ring in their work on inhibitors of dihydrofolate reductase, but they did not explore this further.12 In 2000, a series of rigid dihydrodiaminotriazines with one bulky substituent at the 6-position, designed with the aid of molecular modelling, displayed promising activity against A16V+S108T mutants.5,13 In light of this, the initial series of novel analogues designed in this study contained flexible linkers ranging in length from 1 to 4 atoms between the dihydrotriazine and aromatic rings, and all contained a phenyl substituent at the 6-position of the dihydrotriazine ring (5).Our initial approach to the synthesis of cycloguanil analogues 5 involved preparation of a biguanide precursor 6, followed by reaction with a suitably substituted aldehyde (Scheme 1).14 However, this approach resulted in the formation of a mixture of products in most cases, with the isomeric compound 7 predominating.15 The isomeric compounds were found to have only moderate activity against P. falciparum in vitro (0.991–49.8 μM).15
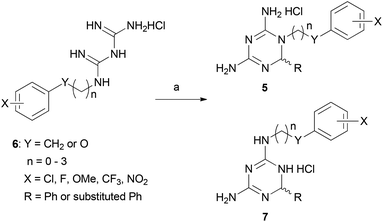 | ||
| Scheme 1 Reagents and conditions: (a) RCHO, 1,4-dioxane, conc. HCl (cat), 100 W, 85 °C, 30 min.15 | ||
We therefore modified our approach to avoid the formation of isomeric mixtures of products by first forming a substituted imine and reacting this with dicyandiamide (Scheme 2).16 To this end, suitably substituted amines 8 were treated with aromatic aldehydes in the presence of molecular sieves or the acidic clay Montmorillonite K-10 to afford substituted imines 9 (Scheme 2). Alternatively, imines 9 could be prepared neat at 100 °C, followed by treatment with 1,4-dioxane and molecular sieves. The imines prepared were then converted to hydrochloride salts by treatment with anhydrous HCl gas, followed by reaction with dicyandiamide to afford the desired cycloguanil analogues 5 in low yields. The reaction sequence was most commonly carried out in a one-pot multi-step fashion using microwave irradiation, however, conventional heating could also be employed both in the formation of the imine and in the final ring closing step. Amines 8 with n = 0 or 1 and Y = CH2 were commercially available, while those with n = 2–3 were prepared from suitably substituted phenols (affording 8 with Y = O) as described previously.15
The initial series of compounds synthesised (Table 1) were assessed for antimalarial activity in vitro in a whole cell P. falciparum assay against a cycloguanil resistant strain (Gambian FCR-3; 3H hypoxanthine incorporation assay). A clear structure–activity relationship became apparent from this initial series of compounds synthesised, with the two compounds bearing a flexible linker of 4 atoms (5g and 5h, entries 7 and 8 of Table 1) exhibiting activity in the low nanomolar range comparable with pyrimethamine (IC50 55.0 nM and 39.8 nM respectively, cf. pyrimethamine IC50 96.2 nM in this assay, entry 10 of Table 1). All other compounds of the series bearing flexible linkers of 1, 2 or 3 atoms only showed activity comparable with cycloguanil against the drug resistant P. falciparum strain (IC50 2.12–7.16 μM; cf. cycloguanil IC50 14.5 μM in this assay, entry 9 of Table 1). Significantly, both 5g and 5h were found to be non-toxic to mammalian cells at therapeutic concentrations, with a selectivity window >700 between cytotoxic and effective concentrations (HeLa cell line, sulforhodamine B (SRB) assay, IC50 39.0 μM for 5g and 33.5 μM for 5h). We later synthesised compounds bearing 5 and 6 atom linkers (general structure 5, n = 4 and n = 5), but these compounds once again only exhibited antimalarial activity in the low micromolar range (2.57–3.98 μM), indicating that the 4 atom linker was essential for potent antimalarial activity.
| Entry | Inhibitor | Pf FCR-3 |
|---|---|---|
IC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (μM) a (μM) |
||
| a Values are expressed as the mean ± SD of triplicate determinations. | ||
| 1 |
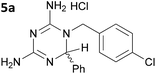
|
6.42 ± 2.24 |
| 2 |
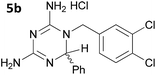
|
4.32 ± 1.19 |
| 3 |
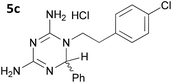
|
7.16 ± 0.63 |
| 4 |
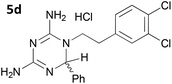
|
4.77 ± 0.30 |
| 5 |
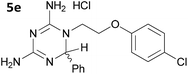
|
4.88 ± 0.69 |
| 6 |
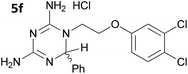
|
2.12 ± 0.75 |
| 7 |
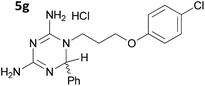
|
0.0550 ± 0.0131 |
| 8 |
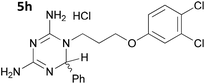
|
0.0398 ± 0.0971 |
| 9 | Cycloguanil | 14.5 ± 2.5 |
| 10 | Pyrimethamine | 0.0962 ± 0.0148 |
| 11 | Chloroquine | 0.0722 ± 0.0118 |
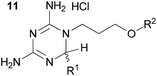
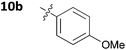
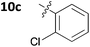
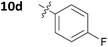
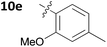
With these promising results in hand, we then embarked on the synthesis of a second series of analogues 10, all of which contained an unsubstituted phenyl substituent at the 6-position of the dihydrotriazine ring and a flexible linker of 4 atoms (Table 2). This series of compounds contained variation in the nature of the aromatic substituent R2 of the flexible linker, and required amines 8 with Y = O, n = 3 and bearing a variety of substituents X. Although commercially available, these amines were also prepared as described previously.15 The final dihydrotriazine hydrochlorides 10a–h were isolated in low yields in general (4%–48%), without optimisation of the reaction conditions at this stage. Gratifyingly, all of the compounds prepared in this series exhibited antimalarial activity in the low nanomolar range, indicating that both electron-withdrawing and electron-donating substituents are tolerated on the aromatic ring R2. The most potent compound in this series (10a, entry 1 of Table 2, IC50 2.66 nM) contained a 2,4-dichlorophenoxy substituent at the end of the flexible linker.
| Entry | ||
|---|---|---|
Pf FCR-3IC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (μM) a (μM) |
||
| a Values are expressed as the mean ± SD of triplicate determinations. | ||
| 1 |
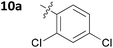
|
0.00266 ± 0.00056 |
| 2 |
|
0.0603 ± 0.0087 |
| 3 |
|
0.171 ± 0.027 |
| 4 |
|
0.0387 ± 0.0163 |
| 5 |
|
0.0465 ± 0.0095 |
| 6 |
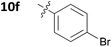
|
0.0241 ± 0.0168 |
| 7 |
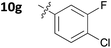
|
0.00955 ± 0.0127 |
| 8 |
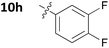
|
0.0908 ± 0.066 |
| 9 | Cycloguanil | 14.5 ± 2.5 |
| 10 | Pyrimethamine | 0.0962 ± 0.0148 |
| 11 | Chloroquine | 0.0722 ± 0.0118 |
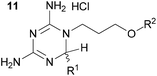
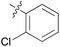
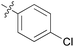
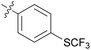
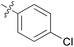
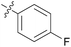
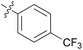
We then explored the effect of substitution on the phenyl ring at the 6-position of the dihydrotriazine, by preparing a third series of analogues 11 (Table 3). This series of analogues contained either a 4-chlorophenoxy or a 3,4-dichlorophenoxy substituent at the end of the flexible linker, with a range of substituted phenyl groups introduced at the 6-position of the dihydrotriazine ring. By comparison, this series of compounds did not perform as well in the in vitro assay, with only 5 of the compounds in this series displaying activity below 100 nM, and 6 of the compounds exhibiting IC50 values in the low micromolar range. In general, compounds bearing a 3,4-dichlorophenoxy substituent at the end of the flexible linker were more potent than the corresponding compound bearing a 4-chlorophenoxy substituent (see for e.g.11tvs.11i or 11uvs.11j, Table 3). Compounds bearing bulkier –CF3, –SCF3 or –OCF3 substituents on the 6-phenyl ring were the least active, most likely due to steric clashes in the DHFR active site (11b, 11h, 11o, 11q, 11r, 11s, Table 3, IC50 2.39–![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) >50 μM). Finally, we prepared an analogue bearing an alkyl flexible chain for comparison with those bearing an oxygen atom (12, entry 22 of Table 3). This compound contained an unsubstituted phenyl ring at the end of the flexible linker, owing to the commercial availability of the precursor 1-bromo-4-phenylbutane. Compound 12 displayed activity in the nanomolar range, although somewhat less potent than the halogenated analogues 10.
>50 μM). Finally, we prepared an analogue bearing an alkyl flexible chain for comparison with those bearing an oxygen atom (12, entry 22 of Table 3). This compound contained an unsubstituted phenyl ring at the end of the flexible linker, owing to the commercial availability of the precursor 1-bromo-4-phenylbutane. Compound 12 displayed activity in the nanomolar range, although somewhat less potent than the halogenated analogues 10.
| Entry | Pf FCR-3 | Entry | Pf FCR-3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | IC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (μM) a (μM) |
R1 | R2 | IC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (μM) a (μM) |
||||
| a Values are expressed as the mean ± SD of triplicate determinations. b IC50 not determined. | |||||||||
| 1 | 11a |
|
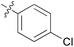
|
0.859 ± 0.606 | 13 | 11m |
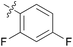
|
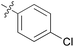
|
0.163 ± 0.050 |
| 2 | 11b |
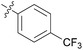
|
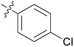
|
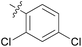
3.37 ± 0.17 | 14 | 11n |
|
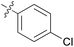
|
2.51 ± 0.67 |
| 3 | 11c |
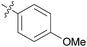
|
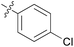
|
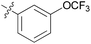
0.0457 ± 0.0172 | 15 | 11o |
|
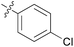
|
>50b |
| 4 | 11d |
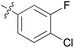
|
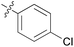
|
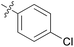
1.59 ± 0.30 | 16 | 11p |
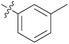
|
|
0.0449 ± 0.0405 |
| 5 | 11e |
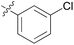
|
|
0.156 ± 0.012 | 17 | 11q |
|
|
2.39 ± 0.03 |
| 6 | 11f |
|
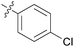
|
0.155 ± 0.048 | 18 | 11r |
|
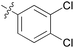
|
4.53 ± 0.63 |
| 7 | 11g |
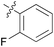
|
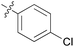
|
0.412 ± 0.080 | 19 | 11s |
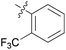
|
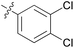
|
3.06 ± 0.04 |
| 8 | 11h |
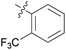
|
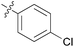
|
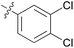
3.24 ± 0.45 | 20 | 11t |
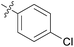
|
|
0.0607 ± 0.0361 |
| 9 | 11i |
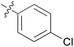
|
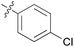
|
0.156 ± 0.129 | 21 | 11u |
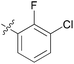
|
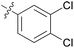
|
0.0977 ± 0.0049 |
| 10 | 11j |
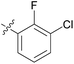
|
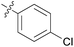
|
0.679 ± 0.221 | 22 | 12 |
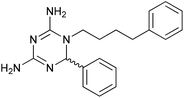
|
0.463 ± 0.059 | |
| 11 | 11k |
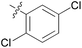
|
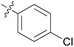
|
0.926 ± 0.128 | |||||
| 12 | 11l |
|
|
0.0772 ± 0.0319 | 23 | Cycloguanil | 14.5 ± 2.5 | ||
| 24 | Pyrimethamine | 0.0962 ± 0.0148 | |||||||
| 25 | Chloroquine | 0.0722 ± 0.0118 | |||||||
These compounds were also assessed for in vitro antimalarial activity against the drug sensitive 3D7 strain using the pLDH assay (Table 4). Similar trends were observed against this strain, with the majority of compounds tested displaying activity in the nanomolar range. The exceptions contained bulky –OCF3 (11o) or –SCF3 (11q) groups on the 6-phenyl ring (Table 4, IC50 1.49 μM and 1.04 μM respectively), as had been observed against the drug resistant FCR-3 strain. Surprisingly, however, the compounds substituted with a –CF3 group on the 6-phenyl ring (11b, 11h, 11r and 11s) that had performed poorly against the FCR-3 strain, showed promising activity against the 3D7 strain bearing wildtype DHFR (Table 4, IC50 66.3 nM–530 nM). Key structure–activity relationships are summarised in Fig. 2. Cytotoxicity of the majority of compounds was tested by SRB assay on the HeLa cell line. Significantly, none of the compounds tested were cytotoxic at nanomolar concentrations, with IC50 values in the micromolar range (Table 4, IC50 10.9–![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) >100 μM).
>100 μM).
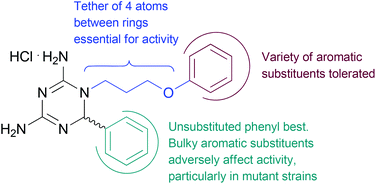 | ||
| Fig. 2 Key structure–activity relationships identified for the 6-aryl-1,6-dihydro-1,3,5-triazine-2,4-diamines prepared. | ||
| Entry | Inhibitor | Pf 3D7 | HeLa |
|---|---|---|---|
IC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (μM) a (μM) |
IC50 (μM) | ||
| a Values are expressed as the mean ± SD of triplicate determinations. b Standard error calculated using Gnuplot. c ND = Not determined. | |||
| 1 | 5g | 0.130 ± 0.027 | 39.0 ± 3.8b |
| 2 | 5h | 0.0183 ± 0.0121 | 33.6 ± 1.6 |
| 3 | 10a | 0.0610 ± 0.0291 | 32.7 ± 2.1 |
| 4 | 10b | 0.0590 ± 0.0014 | >100 |
| 5 | 10c | 0.627 ± 0.174 | 88.8 ± 20.8 |
| 6 | 10d | 0.048 | >100 |
| 7 | 10e | 0.252 ± 0.140 | NDc |
| 8 | 10f | 0.165 ± 0.104 | ND |
| 9 | 10g | 0.0385 ± 0.0176 | ND |
| 10 | 10h | 0.155 ± 0.033 | ND |
| 11 | 11a | 0.290 ± 0.017 | 15.7 ± 2.1 |
| 12 | 11b | 0.0663 ± 0.0085 | 11.0 ± 1.8 |
| 13 | 11c | 0.0493 ± 0.0153 | 25.0 ± 3.3 |
| 14 | 11d | 0.140 ± 0.020 | 13.7 ± 2.1 |
| 15 | 11e | 0.0520 ± 0.0061 | 14.1 ± 2.2 |
| 16 | 11f | 0.687 ± 0.019 | ND |
| 17 | 11g | 0.0783 ± 0.0164 | 24.3 ± 3.0 |
| 18 | 11h | 0.371 ± 0.081 | 18.9 ± 1.6 |
| 19 | 11i | 0.0170 ± 0.0017 | 15.6 ± 2.5 |
| 20 | 11j | 0.143 ± 0.042 | 23.0 ± 1.7 |
| 21 | 11k | 0.340 ± 0.010 | 10.9 ± 0.4 |
| 22 | 11l | 0.463 ± 0.086 | 33.3 ± 4.9 |
| 23 | 11m | 0.150 ± 0.035 | 31.8 ± 1.7 |
| 24 | 11n | 0.193 ± 0.038 | 12.2 ± 1.5 |
| 25 | 11o | 1.49 ± 1.17 | ND |
| 26 | 11p | 0.294 ± 0.142 | ND |
| 27 | 11q | 1.04 ± 0.34 | ND |
| 28 | 11r | 0.109 ± 0.036 | 13.1 ± 1.7 |
| 29 | 11s | 0.530 ± 0.150 | 11.8 ± 0.2 |
| 30 | 11t | 0.122 ± 0.034 | 13.4 ± 1.5 |
| 31 | 11u | 0.0570 ± 0.0157 | 13.6 ± 1.6 |
| 32 | 12 | 0.195 ± 0.074 | ND |
| 33 | Cycloguanil | 0.00413 ± 0.00110 | |
| 34 | Emetine | 0.0202 ± 0.006 | |
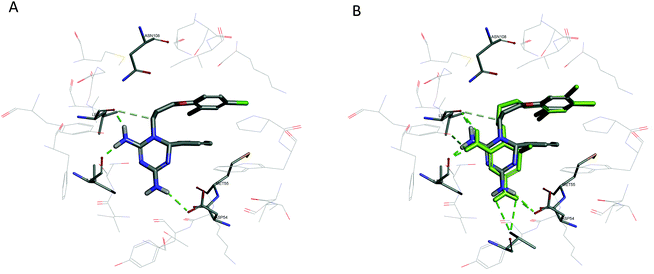
All compounds with an IC50 value below 100 nM in the whole cell in vitro assay against the drug resistant strain (FCR-3) were assessed in a PfDHFR biochemical enzyme assay against both the PfDHFR wild type and the Ala16Val+Ser108Thr double mutant (Table 5). The data obtained from these assays confirm that the compounds act as inhibitors of parasitic DHFR, with all compounds exhibiting Ki values in the low nanomolar range against both the wild type and mutant forms of the enzyme. From our molecular modelling studies, the dihydrotriazines prepared in this study bind in the PfDHFR active site in a similar manner to WR99210 (Fig. 3).
| Entry | Inhibitor | K i Wild type | K i A16VS108T |
|---|---|---|---|
| PfDHFRa (nM) | PfDHFRa (nM) | ||
| a Values are expressed as the mean ± SD of triplicate determinations. | |||
| 1 | 5g | 4.9 ± 0.5 | 8.2 ± 1.1 |
| 2 | 5h | 2.3 ± 0.2 | 4.9 ± 0.7 |
| 3 | 10a | 2.5 ± 0.0 | 6.5 ± 1.1 |
| 4 | 10b | 10.3 ± 2.0 | 15.5 ± 2.4 |
| 5 | 10d | 29.5 ± 5.8 | 25.9 ± 3.8 |
| 6 | 10f | 7.7 ± 1.3 | 14.1 ± 1.4 |
| 7 | 10g | 5.0 ± 0.6 | 11.2 ± 1.6 |
| 8 | 10h | 17.6 ± 1.3 | 29.1 ± 2.5 |
| 9 | 11c | 7.6 ± 1.6 | 10.1 ± 1.3 |
| 10 | 11l | 28.6 ± 1.3 | 37.6 ± 4.7 |
| 11 | 11p | 12.2 ± 0.2 | 12.3 ± 1.1 |
| 12 | 11t | 8.3 ± 2.0 | 12.6 ± 1.0 |
| 13 | 11u | 8.0 ± 0.5 | 9.5 ± 1.6 |
| 14 | Cycloguanil | 1.60 ± 0.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
1518 ± 503![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
| 15 | WR99210 | 0.4 ± 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
0.7 ± 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
Conclusion
In summary, we have designed and synthesised a series of flexible analogues of cycloguanil bearing an aromatic substituent at the 6-position of the dihydrotriazine ring. The compounds displayed potent activity against both drug resistant and drug sensitive forms of P. falciparum in a whole cell in vitro assay, and were shown to act as inhibitors of parasitic DHFR in a biochemical enzyme assay. Good activities against the mutant enzyme (Ala16Val+Ser108Thr DHFR) can be explained by the flexibility of the 5-side chain which can avert steric hindrance introduced by the Ser108Thr mutation, while the 6-aryl substituent, in contrast to the 6,6-dimethyl groups of cycloguanil, can avert steric hindrance caused by the Ala16Val mutation. This is indicative of the potential application of these compounds as antimalarial leads for further development.Experimental section
Chemistry
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone (9
acetone (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (50 ml) and stirred until a solid had formed at the bottom of the flask. The desired product was filtered and washed with diethyl ether.
1) (50 ml) and stirred until a solid had formed at the bottom of the flask. The desired product was filtered and washed with diethyl ether.
Alternatively, Montmorillonite K-10 (150 mg) was added to a microwave tube containing a solution of amine 8 (1.50 mmol) and the appropriate aldehyde (3 eq., 4.50 mmol) in 1,4-dioxane (2 ml). The contents were irradiated with microwave energy (power = 150 W, temperature = 100 °C, time = 30 min). The mixture was allowed to cool to ambient temperature and dried with MgSO4. Hydrogen chloride gas was bubbled through the mixture until saturation. Dicyandiamide (1.2 eq., 1.75 mmol) dissolved in a minimum volume of anhydrous DMF was added to the above mixture. The tube was inserted into the microwave synthesiser and was irradiated with microwave energy (power = 150 W, temperature = 100 °C, time = 30 min). After cooling to ambient temperature, the contents were poured into diethyl ether. The resulting suspension was then filtered through Celite. The solids were washed copiously with diethyl ether. Thereafter methanol was used to elute the desired product.
The following compounds were prepared using these methods:
Biology
3H Hypoxanthine incorporation assay. The chloroquine-resistant Gambian FCR-3 strain was cultured in vitro according to the method described by Jensen and Trager18 and van Zyl et al.19 For experimental purposes the cultures were synchronized with 5% D-sorbitol when the parasites were in the ring stage.20 The antimalarial activity of the various compounds was determined using the tritiated hypoxanthine incorporation assay.21 The parasite suspension, consisting of predominately the ring stage, was adjusted to a 0.5% parasitaemia and 1% haematocrit in hypoxanthine-free RPMI-1640 culture medium with 10% human plasma and exposed to the 6 to 7 concentrations of each compound for a single cycle of parasite growth. All assays were carried out using untreated parasites and uninfected red blood cells as controls. Labeled 3H-hypoxanthine was added after 24 h and the parasitic 3H-DNA harvested after a further 24 h incubation period at 37 °C in a humidified environment. The concentration that inhibited 50% of parasite growth (IC50 value) was determined from the sigmoidal log dose response curve generated by the Enzfitter® and GraphPad Prism® software.
pLDH assay. Three-fold serial dilutions of the test compounds were incubated with 3D7 strain P. falciparum parasites (2% parasitaemia, 1% haematocrit) in 96-well plates containing RPMI 1640 medium supplemented with 25 mM HEPES, 0.5% (w/v) Albumax II, 22 mM glucose, 0.65 mM hypoxanthine, 0.05 mg mL−1 gentamicin and 1% (v/v) human erythrocytes. Incubations were initiated when parasites were predominantly in the trophozoite stage and continued for 48 h at 37 °C in sealed containers filled with an atmosphere of 5% CO2, 5% O2, 90% N2. Subsequently, a colourimetric assay for parasite lactate dehydrogenase (pLDH) activity in individual wells was carried out.22 Twenty μL of culture was removed from the individual wells and transferred to a second 96-well plate containing 125 μL per well pLDH assay reagent (44 mM Tris buffer, pH 9, containing 0.18 M L-lactic acid, 0.13 mM acetylpyridine adenine dinucleotide, 0.39 mM nitrotetrazolium blue chloride, 0.048 mM phenazine ethosulphate and 0.16% (v/v) Triton X-100) and incubated at ambient temperature for 10–30 minutes, after which Abs620 was measured in a Tecan Infinite F500 multiwell plate reader. Absorbance values were used to calculate percentage parasite viability relative to control wells containing untreated parasite cultures. IC50 values for individual compounds were calculated from plots of % viability vs. log(concentration) by non-linear regression using GraphPad Prism®.
Cytotoxicity assay. HeLa cells were plated in 96 well plates at a density of 7 × 103 cells per well in EMEM medium containing 5% fetal bovine serum, 2 mM L-glutamine and 50 μg ml−1 gentamicin and incubated overnight at 37 °C in a 5% CO2 incubator. Three-fold serial dilutions of test compounds were added and incubation was continued for a further 48 h. Remaining cell biomass in the wells was determined using a sulforhodamine B (SRB) assay.23 Cells were fixed by removing the medium and briefly adding cold 50% trichloroacetic acid, followed by washing in water and staining with 0.4% (w/v) SRB in 1% (v/v) acetic acid. Unbound stain was removed by washing in 1% acetic acid, after which 10 mM Tris base was added to dissolve bound SRB. The SRB solutions were transferred to a duplicate 96 well plate and Abs540 measured in a Tecan Infinite F500 plate reader. Absorbance readings were converted to % cell viability relative to untreated control wells and used to generate sigmoidal log dose response curves and calculate IC50 values using GraphPad Prism® software.
Enzyme assays and inhibition by analogues. Wild-type and A16V+S108T mutant PfDHFRs were prepared as described earlier.17 The activity of PfDHFR was determined spectrophotometrically. Briefly, the reaction (200 μL) contained 1× DHFR buffer (50 mM TES, pH 7.0, 75 mM β-mercaptoethanol, 1 mg mL−1 bovine serum albumin), 100 μM dihydrofolate, 100 μM NADPH, and PfDHFR enzyme (0.001–0.005 units) of the affinity-purified enzymes. Inhibition of the enzymes by cycloguanil and its analogues was carried out in the presence of varying concentrations of the inhibitor. The Ki values of the inhibitors against the enzymes was determined by fitting to the equation IC50 = Ki(1 + ([S]/Km)), where IC50 is the concentration of inhibitor which inhibits 50% of the enzyme activity under the standard assay condition and Km is the Michaelis constant for dihydrofolate.13,24
Acknowledgements
This research was supported by Parliamentary Grant funding at the CSIR, South Africa. The authors would like to thank Dr P. Steenkamp for HRMS analysis and purity determination by UPLC of all final compounds. We would like to thank NSTDA's Cluster Program Management for financial support to SK.Notes and references
- WHO World Malaria Report 2015, accessed 1 March 2016, http://www.who.int/malaria/publications/world_malaria_report_2015/en/.
- J. Hemingway, H. Ranson, A. Magill, J. Kolaczinski, C. Fornadel, J. Gimnig, M. Coetzee, F. Simard, D. K. Roch, C. Kerah Hinzoumbe, J. Pickett, D. Schellenberg, P. Gething, M. Hoppé and N. Hamon, Lancet, 2016, 385, 1785 CrossRef.
- A. Nzila, S. A. Ward, K. Marsh, P. F. G. Sims and J. E. Hyde, Trends Parasitol., 2005, 21, 292–298 CrossRef CAS PubMed; J. E. Hyde, Acta Trop., 2005, 94, 191 CrossRef PubMed.
- I. M. Kompis, K. Islam and R. L. Then, Chem. Rev., 2005, 105, 593 CrossRef CAS PubMed; J. E. Salcedo-Sora and S. A. Ward, Mol. Biochem. Parasitol., 2013, 188, 51 CrossRef PubMed.
- G. Rastelli, W. Sirawaroporn, P. Sompornpisut, T. Vilaivan, S. Kamchonwongpaisan, R. Quarrell, G. Lowe, Y. Thebtaranonth and Y. Yuthavong, Bioorg. Med. Chem., 2000, 8, 1117 CrossRef CAS PubMed.
- T. Mita, K. Tanabe and K. Kita, Parasitol. Int., 2009, 58, 201 CrossRef CAS PubMed; A. Heinberg and L. Kirkman, Ann. N. Y. Acad. Sci., 2015, 1342, 10 CrossRef PubMed.
- A. G. E. Childs and C. Lambros, Ann. Trop. Med. Parasitol., 1986, 80, 177 Search PubMed.
- C. Sirichaiwat, C. Intaraudom, S. Kamchonwongpaisan, J. Vanichtanankul, Y. Thebtaranonth and Y. Yuthavong, J. Med. Chem., 2004, 47, 345 CrossRef CAS PubMed; Y. Yuthavong, B. Tarnchompoo, T. Vilaivan, P. Chitnumsub, S. Kamchongwongpaisan, S. A. Charman, D. N. McLennan, K. L. White, L. Vivas, E. Bongard, C. Thongphanchang, S. Taweechai, J. Vanichtanankul, R. Rattanajak, U. Arwon, P. Fantanuzzi, J. Yuvaniyama, W. N. Charman and D. Matthews, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 16823 CrossRef PubMed.
- J. Yuvaniyama, P. Chitnumsub, S. Kamchonwongpaisan, J. Vanichtanankul, W. Sirawaroporn, P. Taylor, M. D. Walkinshaw and Y. Yuthavong, Nat. Struct. Biol., 2003, 10, 357 CrossRef CAS PubMed.
- A. L. Rousseau, D. Gravestock and A. C. U. Lourens, PCT Int. Appl, WO2011018742A120110217, 2011 Search PubMed.
- Docking studies were carried out with Accelrys (now Biovia) Discovery Studio 2.0, using CDocker. The DHFR-TS receptors were obtained from the RCSB PDB: 1J3I (WT) and 1J3K (quadruple mutant) complexed with WR99210, NADPH and dUMP (at the thymidylate synthase (TS) site).
- B. R. Baker and B.-T. Ho, J. Heterocycl. Chem., 1965, 2, 72 CrossRef CAS.
- Y. Yuthavong, T. Vilaivan, N. Chareonsethakul, S. Kamchonwongpaisan, W. Sirawaraporn, R. Quarrell and G. Lowe, J. Med. Chem., 2000, 43, 2738 CrossRef CAS PubMed.
- E. J. Modest, J. Org. Chem., 1956, 21, 1 CrossRef CAS; E. J. Modest and P. Levine, J. Org. Chem., 1956, 21, 14 CrossRef.
- D. Gravestock, A. L. Rousseau, A. C. U. Lourens, S. S. Moleele, R. L. Van Zyl and P. A. Steenkamp, Eur. J. Med. Chem., 2011, 46, 2022 CrossRef CAS PubMed.
- H. Newman and E. L. Moon, J. Org. Chem., 1964, 29, 2061 CrossRef CAS.
- J. Vanichtanankul, S. Taweechai, C. Uttamapinant, P. Chitnumsub, T. Vilaivan, Y. Yuthavong and S. Kamchonwongpaisan, Antimicrob. Agents Chemother., 2012, 56, 3928 CrossRef CAS PubMed.
- J. B. Jensen and W. Trager, Science, 1976, 193, 674 Search PubMed.
- R. L. van Zyl, S. T. Seatlholo, S. F. van Vuuren and A. M. Viljoen, J. Essent. Oil Res., 2006, 18, 129 CrossRef CAS.
- C. Lambros and P. Vanderberg, J. Parasitol., 1979, 65, 418 CrossRef CAS PubMed.
- R. E. Desjardins, C. J. Canfield, D. J. Haynes and J. D. Chulay, Antimicrob. Agents Chemother., 1979, 16, 710 CrossRef CAS PubMed.
- M. T. Makler, J. M. Ries, J. A. Williams, J. E. Bancroft, R. C. Piper, B. L. Gibbins and D. J. Hinrichs, Am. J. Trop. Med. Hyg., 1993, 48, 739 CAS.
- P. Skehan, R. Storeng, D. Scudiero, A. Monks, J. McMahon, D. Vistica, J. T. Warren, H. Bokesch, S. Kenney and M. R. Boyd, J. Natl. Cancer Inst., 1990, 82, 1107 CrossRef CAS PubMed.
- B. Tarnchompoo, C. Sirichaiwat, W. Phupong, C. Intaraudom, W. Sirawaraporn, S. Kamchonwongpaisan, J. Vanichtanankul, Y. Thebtaranonth and Y. Yuthavong, J. Med. Chem., 2002, 45, 1244 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ob01350c |
| This journal is © The Royal Society of Chemistry 2016 |