Junhao Jia, Ruijiao Chen, Hao Liu, Xiong Li, Yuanliang Jia and Xiaochuan Chen
Org. Biomol. Chem., 2016,14, 7334-7344
DOI:
10.1039/C6OB01064D,
Paper
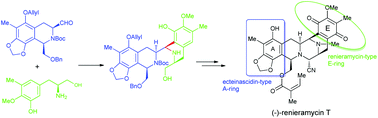
(−)-Renieramycin T, an interesting tetrahydroisoquinolinequinone alkaloid with a novel renieramycin–ecteinascidin mixed framework, is synthesized from the known phenol 16 in 22 steps with 6.2% overall yield. In the convergent route, the key cyclocondensation between the isoquinoline moiety 27 and trisubstituted phenylalaninol 14 is achieved with good selectivity to furnish bistetrahydroisoquinoline 29, which permits a rapid construction of the pentacyclic framework having a fully substituted aromatic A ring.