Iodine-catalysed regioselective thiolation of flavonoids using sulfonyl hydrazides as sulfenylation reagents†
Abstract
Iodine-catalysed regioselective sulfenylation of flavonoid derivatives with sulfonyl hydrazides was developed. Various flavonoid thioethers were obtained in moderate to good yield. The thiolation could be conveniently directed to C-8 for flavone, flavonol, dihydroflavone, and isoflavone derivatives or to C-7 for aurone derivatives by employing the isopropyl ethers of flavonoids bearing free OH groups at the C-5 or C-4 positions.
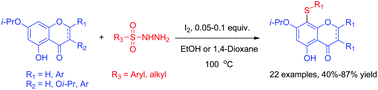

 Please wait while we load your content...
Please wait while we load your content...