A strategy for the synthesis of hydrophobic proteins and glycoproteins
Abstract
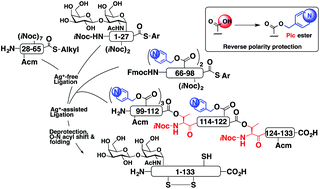
Our synthetic strategy for the hydrophobic glycoprotein is summarized. The reverse-polarity protection strategy, in which the side chain carboxy group is protected as a basic picolyl ester, combined with the O-acylisopeptide method proved to be efficient for the preparation of the hydrophobic glycoprotein by ligation methods, and would be applicable for the synthesis of membrane proteins in the future.
- This article is part of the themed collection: Selective Chemistry with Peptides and Proteins

 Please wait while we load your content...
Please wait while we load your content...