Isocyanates and isothiocyanates as versatile platforms for accessing (thio)amide-type compounds
Abstract
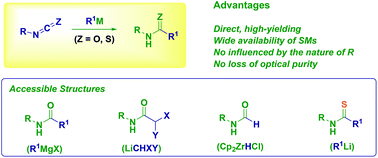
The addition of carbon (Grignard and organolithium reagents) and hydride nucleophiles (Schwartz reagent) to isocyanates and isothiocyanates constitutes a versatile, direct and high yielding approach to the synthesis of functionalized (thio)amide derivatives including haloamides and formamides. The chemoselective delivery of a nucleophilic (eventually configurationally stable) organometallic species to a given iso(thio)cyanate is the crucial parameter for the success of the strategy. Thus, the influence of the factors governing classical methodologies (e.g. dehydrative condensation) such as steric hindrance and electronic properties of the reactants become practically negligible.

 Please wait while we load your content...
Please wait while we load your content...