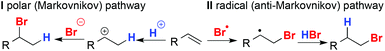Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Scalable anti-Markovnikov hydrobromination of aliphatic and aromatic olefins†
Marzia
Galli
a,
Catherine J.
Fletcher
a,
Marc
del Pozo
b and
Stephen M.
Goldup
*a
aChemistry, University of Southampton, Highfield, Southampton, SO17 1BJ, UK. E-mail: s.goldup@soton.ac.uk
bSchool of Biological and Chemical Sciences, Queen Mary University of London, Mile End Road, London, E1 4NS, UK
First published on 9th May 2016
Abstract
To improve access to a key synthetic intermediate we targeted a direct hydrobromination-Negishi route. Unsurprisingly, the anti-Markovnikov addition of HBr to estragole in the presence of AIBN proved successful. However, even in the absence of an added initiator, anti-Markovnikov addition was observed. Re-examination of early reports revealed that selective Markovnikov addition, often simply termed “normal” addition, is not always observed with HBr unless air is excluded, leading to the rediscovery of a reproducible and scalable initiator-free protocol.
Terminal alkenes are readily converted into valuable synthetic intermediates for metal-mediated cross-coupling reactions by hydro-metallation to give organo-boron1 and other organometallics.2 These reactions typically proceed under steric control to give the primary organometallic, often designated as the “anti-Markovnikov” product, a term that refers back to seminal work done over 140 years ago by Victor Markovnikov on the analogous addition of HI to alkenes.3,4
We recently applied such a hydrometallation-Suzuki approach to the synthesis of bromopyridine 2,5a a key intermediate in the synthesis of mechanically chiral rotaxanes, stabilised reactive organometallic species and interlocked catalysts;5 hydroboration of commodity chemical estragole (1a) with 9-BBN-H followed by an in situ cross coupling with 2,6-dibromopyridine yielded 2 in a concise manner (Scheme 1). However, on scale up we encountered problems with purification due to the borinic acid by-product, which, in addition to the high cost of 9-BBN-H, led us to explore other routes to 2. Accordingly, we explored a Negishi approach employing an organozinc species produced in situ from bromide 3a,6 itself accessed in three steps from cheap and readily available hydroxyphenyl propionic acid 4.7 However, although the Negishi coupling step is efficient and scalable, the three-step synthesis of bromide 3a once again proved cumbersome on scale up.
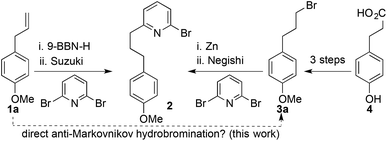 | ||
| Scheme 1 Reported5a,7 and proposed routes to bromopyridine 2. | ||
These issues led us to consider the direct anti-Markovnikov hydrobromination of estragole to produce 3a in order to combine the key advantages of both syntheses. This approach proved extremely successful giving rapid access to 3a and thus 2 in multi-gram quantities. More importantly, as a result of these studies we made an initially surprising observation: even in the absence of added initiators the hydrobromination of 1a proceeds in reasonable selectivity to give the anti-Markovnikov product.
Here we report how this observation led to the rediscovery of simple scalable conditions for synthesis of primary bromides under “initiator free” conditions from alkyl and aryl alkenes. Our results increase the availability of primary bromides directly from feedstock alkene substrates.
The hydrobromination of olefins is generally held to proceed through two competing pathways:8 polar pathway Ivia the most stable carbocation typically resulting in the branched, Markovnikov product, and radical pathway IIvia the most stable radical, resulting in the linear, anti-Markovnikov product (Fig. 1). To favour pathway II, reactions are carried out in apolar solvents in the presence of radical initiators (the “peroxide effect”)9 or under irradiation.10 The Markovnikov and anti-Markovnikov products are also often simply called the “normal” and “abnormal” products respectively.11
Surprisingly, direct synthesis of primary bromides from monosubstituted alkenes by reaction with HBr appears to be a relatively under-used reaction;12 a simple search gave only 330 examples compared with the >48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 such bromides reported.13 We were also surprised to be unable to find anything recognisable as an organic methodological study in which a variety of substrates are screened under the same conditions, presumably because most work on the peroxide effect was carried out in the first half of the 20th century with each paper reporting only a few examples.11 Thus, most recent reports of this transformation are confined to isolated examples as part of a larger synthetic campaign.
000 such bromides reported.13 We were also surprised to be unable to find anything recognisable as an organic methodological study in which a variety of substrates are screened under the same conditions, presumably because most work on the peroxide effect was carried out in the first half of the 20th century with each paper reporting only a few examples.11 Thus, most recent reports of this transformation are confined to isolated examples as part of a larger synthetic campaign.
A brief screen of conditions14,15 identified the use of HBr in PhMe in the presence of AIBN as appropriate, giving 3a in excellent 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 selectivity (Scheme 2).16 A minor drawback of this procedure on larger scales is the relatively high loading (13 mol%) of AIBN required. Unfortunately, attempts to reduce this led to erratic results (see below). However, the excess AIBN could be removed readily simply by filtering the reaction mixture through silica prior to evaporation and applying this procedure allowed us to reliably produce 3a across a range of scales (1–200 mmol) in excellent yield (98%).
3 selectivity (Scheme 2).16 A minor drawback of this procedure on larger scales is the relatively high loading (13 mol%) of AIBN required. Unfortunately, attempts to reduce this led to erratic results (see below). However, the excess AIBN could be removed readily simply by filtering the reaction mixture through silica prior to evaporation and applying this procedure allowed us to reliably produce 3a across a range of scales (1–200 mmol) in excellent yield (98%).
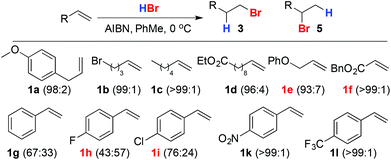 | ||
Scheme 2 Addition of HBr in PhMe to alkenes. Figures in parentheses refer to the selectivity 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. Reagents and conditions: HBr in PhMe (sat.), AIBN (13 mol%), 0 °C, 2 h. 5. Reagents and conditions: HBr in PhMe (sat.), AIBN (13 mol%), 0 °C, 2 h. | ||
Moreover, these conditions proved general for representative monosubstituted aliphatic alkenes (1b–f). The slightly reduced selectivity in the case of allyl ether 1e may be due to anchimeric assistance by the proximal O atom favouring the linear product. To our knowledge there are no previous reports of the direct addition of HBr to aromatic alkenes to give the primary bromide product in high selectivity,17,18 presumably because the aromatic substituent can stabilise the cation formed in the Markovnikov pathway. In keeping with this, poor selectivity was observed in the case of styrene (1g) and this was reduced further in the case of a weakly electron donating p-fluoro substituent (1h). Conversely, a weakly electron-withdrawing p-chloro substituent (1i) led to higher selectivity and strongly electron-withdrawing substituents (1k, 1l) gave excellent selectivity for the linear product.17
Based on these results the reaction of HBr in toluene with AIBN appears general for aliphatic alkenes but only applicable to electron styrenes bearing strongly electron withdrawing substituents. However, during our attempts to reduce the AIBN loading we made an unexpected observation: on small scales (1 mmol), even when no external initiator was added a significant selectivity for primary bromide 3a was still observed, albeit with poor reproducibility. Based on the received wisdom of undergraduate chemistry this result is superficially surprising as, in the absence of added initiators or irradiation, the Markovnikov product is predicted in systems that lack significant electronic bias.19
In order to understand this observation we returned to the early publications in the field, in particular an excellent contemporary review from Walling.11 This revealed a number of interesting points often omitted in recent discussions. Firstly, many of the early investigations of the addition of HBr to alkenes were conducted using the neat alkene, rather than under dilute conditions where the polar pathway is dramatically retarded. Secondly, in order to observe the Markovnikov product, great care was always taken to use extremely pure alkene substrates and exclude oxygen and other adventitious oxidants because, although Markovnikov addition is the rule for HCl and HI, the case of HBr is far more nuanced; even in the presence of vanishingly small quantities of oxidants, anti-Markovikov addition can compete and even dominate in the case of alkenes that are not activated to Markivnikov addition.
Thus our surprise observation was in fact common knowledge when the peroxide effect was first discovered. Perhaps unhelpfully, although Markovnikov's rule is often discussed in the context of hydrobromination, HBr is the only hydrohalous acid in which this outcome is sometimes hard to observe experimentally as anti-Markovnikov addition often competes due to the presence of adventitious oxidants. Indeed, the terms “normal” and “abnormal” addition actually seem to have originally referred to the reactions of HI and HCl in which no peroxide effect is observed and thus the abnormal addition actually refers to the contrast with these products, rather than with that observed in the case of HBr “normally”.
During our literature search, one of the early examples of initiator-free hydrobromination caught our attention. Sherrill and co-workers reported in 1934 that HBr added as a solution in AcOH gave reliable anti-Markovnikov addition to pent-1-ene and hept-1-ene in hexane.20 Sherrill's conditions have been applied only twice in synthesis and the origin of the unusual reaction outcome was not commented upon.21,19a,b These conditions are particularly attractive as the use of a commercially available solution of HBr in AcOH is operationally simpler than using HBr gas to produce an HBr solution.
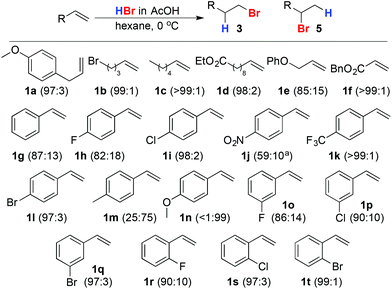
Under Sherrill's original conditions, aliphatic alkenes 1a–f, with the exception of allyl ether 1e, were hydrobrominated in excellent selectivity, comparable to that observed in toluene in the presence of AIBN. Furthermore, these conditions deliver improved selectivity (>80%) for the primary bromide product in the case of styrene itself (1g) and p-fluoro-styrene (1h). Furthermore, even weakly (1i, 1l) electron withdrawing para substituents led to excellent (>95%) selectivity for the primary bromide. Surprisingly, the reaction of p-NO2 styrene (1k) failed to reach completion and gave poor selectivity for reasons that are currently unclear. Styrenes bearing a p-electron donating substituent (1m, 1n) led unsurprisingly predominantly to Markovnikov addition. The reaction is not limited to para-substituted styrenes; meta- (1o–1q) and ortho-halo styrenes (1r–1t) were hydrobrominated in good to excellent selectivity (Scheme 3).
The anti-Markovnikov hydrobromination reaction in hexane under Sherril's conditions is operationally far simpler than those commonly employed in synthesis; the substrate is simply dissolved and the HBr added directly as a commercially available solution in AcOH, removing the need to saturate the reaction solvent with HBr gas or the addition of supplementary initiators, the bi-products of which must be removed after the reaction. The only requirement for the reaction to be reproduced reliably across a range of scales was for air to be passed through a solution of the alkene in hexane prior to the addition of AcOH–HBr. It is worth noting that the observation of spontaneous anti-Markovnikov addition in apolar solvents has previously led to Mahrouz and co-workers22 and Sergeev et al.23 to independently propose alternative mechanisms to the standard Markovnikov and anti-Markovnikov models (Fig. 1). Our results clearly support the peroxide effect orthodoxy; on larger scales, reactions in hexane–AcOH are enhanced when air is introduced intentionally, suggesting that O2 initiates the process by oxidising HBr to produce Br radicals.
Finally, to demonstrate the advantage of the initiator-free hydrobromination process we returned to our original problem, the simple, rapid and concise synthesis of bromopyridine 2 (Scheme 4). Hydrobromination of 1a gave 23 g (90%) of 3a. Importantly no purification was required beyond simple aqueous workup. Subsequent formation of the primary organozinc of 3a under Huo's conditions6 and cross-coupling with 2,6-dibromopyridine yielded 14 g of key intermediate 2 (60% yield; 43% based on estragole over two steps), demonstrating that this approach to primary bromides from feedstock alkene substrates produces material in suitable purity for direct application in cross-coupling reactions.
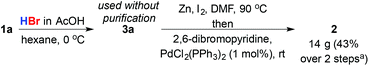 | ||
| Scheme 4 Synthesis of alkyl pyridine 2 from estragole. aBased on 1a; 60% yield based on 2,6-dibromopyridine. | ||
Conclusions
In conclusion, we have developed simple, scalable and high yielding conditions for the selective direct anti-Markovnikov hydrobromination of monosubstituted terminal aliphatic and, for the first time, aromatic alkenes. The omission of initiators such as AIBN or benzoylperoxide removes the need for purification of the products, allowing them to be taken forward directly in further synthetic manipulations. To be clear, we achieved this not by discovering new conditions but by investigating and generalising a previously reported but largely forgotten procedure from Sherrill and co-workers. That this procedure has remained largely ignored for so long is surprising given its synthetic utility and may be in part due to the counterintuitive nature of the conditions, in that no obvious initiator is added, combined with the lack of previous methodological investigations. The results presented here should increase the synthetic availability of primary bromides as synthetic intermediates derived from feedstock monosubstituted terminal alkenes.Acknowledgements
We thank Fluorochem for the gift of reagents and the EPSRC (EP/L016621/1), Queen Mary, University of London and the University of Southampton for funding. SMG is a Royal Society Research Fellow.Notes and references
- For examples see: (a) H. C. Brown, Hydroboration, W. A. Benjamin, New York, 1962 Search PubMed; (b) H. C. Brown, (with techniques by G. W. Kramer, A. B. Levy, M. M. Midland), Organic Syntheses via Boranes, Wiley-Interscience, New York, 1975 Search PubMed.
- For a recent review on the hydrometallation of alkenes in the context of asymmetric conjugate addition see: R. M. Maksymowicz, A. J. Bissette and S. P. Fletcher, Chem. – Eur. J., 2015, 21, 5668 CrossRef CAS PubMed.
- (a) W. Markownikoff, Ann. der Chemie und Pharm., 1870, 153, 228 CrossRef; (b) V. V. Markovnieoff, C. R. Hebd. Seances Acad. Sci., 1875, 81, 668 Search PubMed.
- It has been suggested that Markovnikov's “rule” was the result of inspired guess-work rather than significant experimental evidence. For an interesting account see: P. Hughes, J. Chem. Educ., 2006, 83, 1152 CrossRef CAS.
- (a) R. J. Bordoli and S. M. Goldup, J. Am. Chem. Soc., 2014, 136, 4817 CrossRef CAS PubMed; (b) J. Winn, A. Pinczewska and S. M. Goldup, J. Am. Chem. Soc., 2013, 135, 13318 CrossRef CAS PubMed; (c) M. Galli, J. E. M. Lewis and S. M. Goldup, Angew. Chem., Int. Ed., 2015, 54, 13545 CrossRef PubMed; (d) J. E. M. Lewis, R. J. Bordoli, M. Denis, E. A. Neal, C. Fletcher, M. Galli, E. Rochette and S. M. Goldup, Chem. Sci., 2016, 7, 3154 RSC.
- S. Huo, Org. Lett., 2003, 5, 423 CrossRef CAS PubMed.
- V. Aucagne, J. Berná, J. D. Crowley, S. M. Goldup, K. D. Hänni, D. A. Leigh, P. J. Lusby, V. E. Ronaldson, A. M. Z. Slawin, A. Viterisi and D. B. Walker, J. Am. Chem. Soc., 2007, 129, 11950 CrossRef CAS PubMed.
- For example see: J. Clayden, N. Greeves and S. Warren, Organic Chemistry, Oxford University Press, Oxford, 2nd edn, 2012, p. 433 Search PubMed.
- M. S. Kharasch, C. Hannum and M. Gladstone, J. Am. Chem. Soc., 1934, 56, 244 CrossRef CAS.
- W. E. Vaughan, F. F. Rust and T. W. Evans, J. Org. Chem., 1942, 7, 477 CrossRef CAS.
- For an excellent contemporary review of the early work on the addition of hydrohalous acids to alkenes and the peroxide effect, including the observation of anti-Markovnikov addition in the absence of added initiators, see: F. R. Mayo and C. Walling, Chem. Rev., 1940, 27, 351 CrossRef CAS.
- Pseudo-hydrobromination by hydrometallation followed by oxidative bromination has also been demonstrated. See: (a) G. W. Kabalka, K. A. R. Sastry, H. C. Hsu and M. D. Hylarides, J. Org. Chem., 1981, 46, 3113 CrossRef CAS; (b) H. C. Brown and C. F. Lane, Tetrahedron, 1988, 44, 2763 CAS; (c) J. Schwartz and J. A. Labinger, Angew. Chem., Int. Ed. Engl., 1976, 15, 333–340 CrossRef.
- A search using the Reaxys database with the criteria below provides 330 reactions when limited to those that use HBr as a reactant. By comparison, a search for primary bromides of this form suggest >46
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 such compounds are known.
000 such compounds are known.
. - We selected as our starting point the procedure reported by Bloodworth and Mitchel: A. J. Bloodworth, T. Melvin and J. C. Mitchell, J. Org. Chem., 1988, 53, 1078 CrossRef CAS.
- Unfortunately, although the desired conversion of estragole to 3a has previously been reported, detailed experimental conditions were not provided: J. Delobelle, M. Fetizon, P. Baranger, J. Schalbar and M. J. Trefouel, C. R. Hebd. Seances Acad. Sci., 1957, 244, 2402 CAS.
- The majority of literature procedures call for HBr gas, readily produced by reaction of Br2 with tetraline, to be passed through the reaction mixture with all other components present. However, we found this led to poor reproducibility, presumably due to poor control over the concentration of HBr. Pre-saturation of the solvent with HBr prior to addition of the substrate removed this limitation. Furthermore, the solution of HBr in toluene could be stored for up to one month in the freezer.
- Kharasch and co-workers reported the hydrobromination of styrene in dilute pentane solution with debenzoyl peroxide to give an 80
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 ratio in favour of the primary
bromide. Unfortunately, detailed conditions were not provided:
(a) C. Walling, M. S. Kharasch and F. R. Mayo, J. Am. Chem. Soc., 1939, 61, 2693 CrossRef CAS The only other reliable report of the direct hydrobromination of a styrene derivative to give the primary bromide involved pentachloro or tetrachloro-benzenes which have significant electronic bias towards to the primary product.19c.
20 ratio in favour of the primary
bromide. Unfortunately, detailed conditions were not provided:
(a) C. Walling, M. S. Kharasch and F. R. Mayo, J. Am. Chem. Soc., 1939, 61, 2693 CrossRef CAS The only other reliable report of the direct hydrobromination of a styrene derivative to give the primary bromide involved pentachloro or tetrachloro-benzenes which have significant electronic bias towards to the primary product.19c. - Instead of direct hydrobromination, hydrometallation followed by oxidative bromination or oxidation to the alcohol with subsequent conversion to bromide is commonly employed in the synthesis of primary homo-benzyl bromides from styrenes. For selected examples see: (a) E. M. Zippi, H. Andres, H. Morimoto and P. G. Williams, Synth. Commun., 1994, 24, 1037 CrossRef CAS; (b) P. L. Ornstein, M. B. Arnold, N. K. Allen, T. Bleisch, P. S. Borromeo, C. W. Lugar, J. D. Leander, D. Lodge and D. D. Schoepp, J. Med. Chem., 1996, 39, 2219 CrossRef CAS PubMed; (c) M. D. Erion, S. R. Kasibhatla, B. C. Bookser, P. D. Van Poelje, M. R. Reddy, H. E. Gruber and J. R. Appleman, J. Am. Chem. Soc., 1999, 121, 308 CrossRef CAS; (d) S. R. Kasibhatla, B. C. Bookser, G. Probst, J. R. Appleman and M. D. Erion, J. Med. Chem., 2000, 43, 1508 CrossRef CAS PubMed; (e) S. R. Kasibhatla, B. C. Bookser, W. Xiao and M. D. Erion, J. Med. Chem., 2001, 44, 613 CrossRef CAS PubMed; (f) J. Lee, S. Y. Kim, S. Park, J.-O. Lim, J.-M. Kim, M. Kang, J. Lee, S.-U. Kang, H.-K. Choi, M.-K. Jin, J. D. Welter, T. Szabo, R. Tran, L. V. Pearce, A. Toth and P. M. Blumberg, Bioorg. Med. Chem., 2004, 12, 10559 Search PubMed; (g) G. Médard, Tetrahedron, 2014, 70, 186 CrossRef; (h) T. Miyazaki, M. Shibahara, J. Fujishige, M. Watanabe, K. Goto and T. Shinmyozu, J. Org. Chem., 2014, 79, 11440 CrossRef CAS PubMed.
- When the alkene substituent is strongly electron withdrawing, such as in the case of conjugated carbonyl compounds, anti-Markovnikov addition is the expected outcome. For selected examples see: (a) J. B. Christensen and A. Schluter, Org. Prep. Proced. Int., 1994, 26, 355–357 CrossRef CAS; (b) T. Kaku, T. Hitaka, A. Ojida, N. Matsunaga, M. Adachi, T. Tanaka, T. Hara, M. Yamaoka, M. Kusaka, T. Okuda, S. Asahi, S. Furuya and A. Tasaka, Bioorg. Med. Chem., 2011, 19, 6383–6399 CrossRef CAS PubMed; (c) S. D. Ross, M. Markarian, H. H. Young and M. Nazzewski, J. Am. Chem. Soc., 1950, 72, 1133 CrossRef CAS.
- (a) M. L. Sherrill, K. E. Mayer and G. F. Walter, J. Am. Chem. Soc., 1934, 56, 926 CrossRef CAS; M. L. Sherrill, K. E. Mayer and G. F. Walter. Sherrill and Kharasch publicly disagreed on the origin of the unexpected selectivity see: (b) M. S. Kharasch, J. A. Hinckley and M. M. Gladstone, J. Am. Chem. Soc., 1934, 56, 1642 CrossRef CAS; (c) M. L. Sherrill, J. Am. Chem. Soc., 1934, 56, 1645 CrossRef CAS.
- (a) C. M. Moser and M. Paulshock, J. Am. Chem. Soc., 1950, 72, 5419 CrossRef CAS; (b) C. S. Lancefield, L. Zhou, T. Lébl, A. M. Z. Slawin and N. J. Westwood, Org. Lett., 2012, 14, 6166 CrossRef CAS PubMed.
- R. Barlet, A. Alagui, O. Bouab and M. Mahrouz, Actual. Chim., 1999, 2, 26 Search PubMed.
- G. B. Sergeev, N. F. Stepanov, I. A. Leensov, V. V. Smirnov, V. I. Pupyshev, L. A. Tyurina and M. N. Mashyanov, Tetrahedron, 1982, 38, 2585 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental procedures. See DOI: 10.1039/c6ob00692b |
| This journal is © The Royal Society of Chemistry 2016 |