Open Access Article
Open Access ArticleExploiting the σ-phylic properties of cationic gold(I) catalysts in the ring opening reactions of aziridines with indoles†
Elisabetta
Rossi
*,
Giorgio
Abbiati
,
Monica
Dell'Acqua
,
Marco
Negrato
,
Andrea
Paganoni
and
Valentina
Pirovano
Dipartimento di Scienze Farmaceutiche – Sezione di Chimica Generale e Organica “A. Marchesini”, Università degli Studi di Milano, Via G. Venezian, 21 – 20133 Milano, Italy. E-mail: elisabetta.rossi@unimi.it
First published on 25th May 2016
Abstract
A study on the SN2-type ring opening reactions of aziridines with indoles as nucleophiles is reported. Under gold(I) catalysis a great variety of tryptamine derivatives were prepared in good to excellent yields with complete stereocontrol when chiral aziridines were used. We demonstrated that cationic gold(I) catalysts are superior Lewis acids to the previously reported group 3, 12 and 13 metals in terms of catalyst loading and reaction yields. Moreover, complete regioselectivity was observed for 2-phenyl-N-tosylaziridine; whereas, regioselectivity up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio was observed with 2-methyl-N-tosylaziridine. Finally, a preliminary study on the dearomatization reactions giving rise to pyrroloindolines is also reported.
1 ratio was observed with 2-methyl-N-tosylaziridine. Finally, a preliminary study on the dearomatization reactions giving rise to pyrroloindolines is also reported.
Introduction
Aziridine nucleophilic ring-opening reactions have been recognized for a long time as a precious and reliable tool to introduce a CCN motif in a huge number of substrates.1 In the specific case of the indole nucleus, regioselective nucleophilic ring-opening of aziridines led to the formation of tryptamine derivatives, chemically synthesized or naturally occurring drugs/alkaloids with well-defined and significant biological effects.2 However, reported examples with indoles as nucleophiles are relatively rare and involve aziridines bearing an electron withdrawing group at nitrogen (activated aziridines) under (Lewis) acidic catalysis.3 In particular, the regiochemical outcome for reactions involving C2 monosubstituted aziridines largely depends on the substitution pattern (Scheme 1).For example, indoles add regioselectively at the benzylic position of C2 aryl substituted aziridines under LiClO4,3h and Sc(OTf)3/Zn(OTf)2 catalysis,3a or in the presence of an excess of BF3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3i (Scheme 1, eqn (1)). Moving to alkyl substituted aziridines, mixtures of both conceivable regioisomers are isolated using Zn(OTf)2
3i (Scheme 1, eqn (1)). Moving to alkyl substituted aziridines, mixtures of both conceivable regioisomers are isolated using Zn(OTf)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3c as a catalyst, and only one report of regioselective reaction in the presence of an excess of BF3 has been reported until now3i (Scheme 1, eqn (2)). Besides, aziridines 2-carboxylate have been involved in the synthesis of tryptophan derivatives using 1 equivalent of Sc(ClO4)3,3j Sc(OTf)3
3c as a catalyst, and only one report of regioselective reaction in the presence of an excess of BF3 has been reported until now3i (Scheme 1, eqn (2)). Besides, aziridines 2-carboxylate have been involved in the synthesis of tryptophan derivatives using 1 equivalent of Sc(ClO4)3,3j Sc(OTf)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3b or Y(OTf)3
3b or Y(OTf)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3d as a promoter (Scheme 1, eqn (3)). From the data reported in Scheme 1, it should be noted that working with chiral C2 substituted aziridines, enantiomeric pure compounds can be obtained, suggesting the involvement of a SN2-type mechanism for the ring-opening reaction.3i,c Clearly, enantiomeric pure derivatives have been obtained in C3 selective ring opening reactions of enantiomeric pure C2 substituted aziridines.3b,d,j Furthermore, several of the reported reactions suffer from some drawbacks, i.e., they are performed in the presence of at least a stoichiometric amount of Lewis acids, require an excess of aziridine/indole substrates and/or are limited in scope.
3d as a promoter (Scheme 1, eqn (3)). From the data reported in Scheme 1, it should be noted that working with chiral C2 substituted aziridines, enantiomeric pure compounds can be obtained, suggesting the involvement of a SN2-type mechanism for the ring-opening reaction.3i,c Clearly, enantiomeric pure derivatives have been obtained in C3 selective ring opening reactions of enantiomeric pure C2 substituted aziridines.3b,d,j Furthermore, several of the reported reactions suffer from some drawbacks, i.e., they are performed in the presence of at least a stoichiometric amount of Lewis acids, require an excess of aziridine/indole substrates and/or are limited in scope.
On the other hand, indole is one of the most extensively explored heterocyclic nuclei. A plethora of synthetic methodologies have been developed for the assembly of the indole core4 as well as for the functionalization of the preformed nucleus.5 This latter goal has been mainly achieved on the pyrrole moiety with electrophiles, exploiting the enamine-type reactivity at the C3 carbon atom and the nucleophilic properties of the nitrogen atom, and, to a lesser extent, with nucleophiles, reversing the natural reactivity by the insertion of suitable substituents at nitrogen or at C2/C3 positions.
Other interconnected and fascinating research areas encompass the dearomative manipulation of the preformed indole ring6 for the synthesis of the indolenine core and the cyclization/cycloaddition reactions for the synthesis of polycyclic indole derivatives,4e,h,k,7 either common scaffolds in naturally occurring and bioactive compounds. Over the last few years, the effectiveness of all these transformations has been notably enhanced through the development of new catalytic and organocatalytic processes.
Considering these results and in step with our latest research reports on the synthesis of polycyclic indoles8 and indole derivatives9 under gold catalysis, we recognized gold-activated aziridines as suitable electrophiles for the simple functionalization and the dearomative reactions of indoles for the synthesis of β-indolylamines and fused pyrrolo[2,3-b]indoles, respectively (Scheme 2).
Formation of stable complexes via N-coordination of aziridines with in situ generated triphenylphosphine gold(I) triflate (AuPPh3OTf) has been recently described by Lorenz and co-workers.10 These complexes are quite stable and can be isolated and characterized under standard laboratory conditions. Moreover, in 2007 Wu and co-workers published a gold(III) chloride/silver triflate catalyzed ring-opening reaction of aziridines with furan and electron-rich arenes.11 Thus, in the presence of 1–5% of AuCl3 and 3–15% of AgOTf in nitromethane at room temperature, the reaction affords the corresponding β-aryl(furyl)amines in good to excellent yields. Moreover, several papers dealing with the σ-phylic properties of gold species have been recently published.8a,12
Results
We started our investigations testing the reactivity of indoles 1a and 1h with racemic 2-phenyl-N-tosylaziridine (2a), under conventional Lewis acid, organic acid and gold catalyses. A comprehensive review of the reaction conditions we tested is summarized in Table 1.| Entrya | Indole | Eq. of 2a | Catalyst (mol%) | Solvent | T, °C | Time, h | Yield, % |
|---|---|---|---|---|---|---|---|
| a Entries 1–3, 5–14: reaction conditions: to a solution of indole (0.3 mmol) and aziridine (n equiv.) in the appropriate solvent (2 mL, 0.15 M), a catalyst was added and the mixture was stirred for the stated time and temperature. Entry 4, see ref. 3i. b BDHP = 1,1′-binaphthyl-2,2′-diyl hydrogenphosphate. | |||||||
| 1 | 1a | 1.1 | In(OTf)3 (15 mol%) | DCM | rt, 40 | 20 | 42 |
| 2 | 1a | 1.1 | Sc(OTf)3 (15 mol%) | DCM | rt, 40 | 20 | 21 |
| 3 | 1h | 1.5 | Zn(NTf2)2 (15 mol%) | DCE | rt, 60 | 20 | 48 |
| 4 | 1a | 0.6 | BF3·OEt2 (1.5 eq.) | DCM | −20 | 0.2 | 54 |
| 5 | 1h | 1.5 | TfOH (5 mol%) | DCE | rt | 1.5 | 51 |
| 6 | 1h | 1.5 | BDHP (5 mol%)b | DCE | 80 | 20 | Traces |
| 7 | 1a | 1.1 | AuCl3 (5 mol%), AgOTf (15 mol%) | CH3NO2 | rt | 24 | 23 |
| 8 | 1h | 1.5 | AgOTf (5 mol%) | DCM | rt, 40 | 20 | 50 |
| 9 | 1a | 1.5 | AgNTf2 (5 mol%) | DCE | 80 | 24 | 45 |
| 10 | 1h | 1.5 | [Au(PPh3)OTf] (5 mol%) | DCE | 80 | 0.5 | 61 |
| 11 | 1h | 1.5 | [Au(IPr)SbF6] (5 mol%) | DCE | 80 | 0.5 | 79 |
| 12 | 1h | 1.5 | [Au(JohnPhos)NTf2] (5 mol%) | DCE | 80 | 0.5 | 87 |
| 13 | 1h | 1.5 | [Au(PPh3)NTf2] (5 mol%) | DCE | 80 | 0.5 | 80 |
| 14 | 1a | 1.1 | [Au(JohnPhos)NTf 2 ] (5 mol%) | DCE | 80 | 4.5 | 98 |
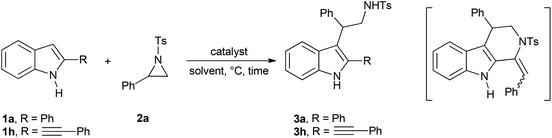
We choose to survey the reactivity of 2-alkynylindole 1h, besides simple and commercially available 1a, to verify the feasibility of a domino process involving nucleophilic addition of an indole to an aziridine and subsequent hydroamination of the triple bond (Table 1, compound in brackets). However, under the tested reaction conditions, this compound has never been isolated or detected in the crude reaction mixtures. When the reaction works toward the formation of the desired compounds 3, the isolated regioisomers arose from the C2 selective ring-opening of the aziridine, compounds 3a and 3h. The structure of 3a was elucidated via 1D and 2D NMR experiments.13 Initially, for comparison, we tested several conventional Lewis acids under catalytic conditions (15 mol%, entries 1–3). Instead, boron trifluoride was used under the conditions reported in ref. 3i (entry 4). These first attempts, however, led to the formation of the desired product in poor or moderate yields. Similar results were obtained in the presence of trifluoroacetic acid as the catalyst, whereas phosphoric acid (1,1′-binaphthyl-2,2′-diyl hydrogenphosphate) proved essentially ineffective, entries 5 and 6. Thus, gold(III) chloride, in the presence of silver triflate as an activating agent, was tested under the reaction conditions reported by Wu,11 entry 7. In this case, 3a was obtained in 23% yield. 5 mol% silver triflate, silver triflimidate or cationic gold(I) species proved more effective, entries 8–14, and compounds 3a and 3h were isolated in 45–98% yields, with [Au(JohnPhos)NTf2] as the catalyst of choice.
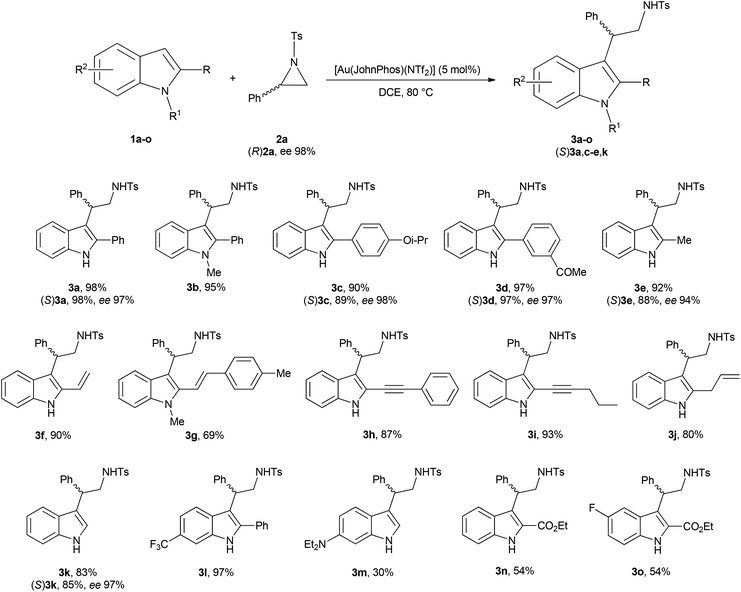
With these results in hand, we initially choose to test the scope of the reaction under the conditions reported in Table 1, entry 14, changing the substituent array on the indole nucleus (Scheme 3).
Product yields ranged from very good to excellent when the reaction was performed with 2-aryl, 2-alkyl, 2-vinyl, 2-alkynyl and 2-allyl indoles (1a, 1c–f, 1h–j), with N-Me protected indoles 1b and 1g and with indole itself (1k). The introduction of an EWG on the indole phenyl moiety is well tolerated (1l), whereas in the presence of an EDG, indole 1m, or when an ethoxycarbonyl group is linked at C2 of the indole nucleus, indoles 1n and 1o, the corresponding tryptamine derivatives 3m–o were obtained in moderate yields. Moreover, the reactions of indoles 1a,c–e,k were repeated in the presence of (R)-2-phenyl-N-tosylaziridine ((R)2a) yielding the corresponding optically active compounds (S)3a,c–e,k in enantiomeric excesses comparable to that of the starting aziridine.
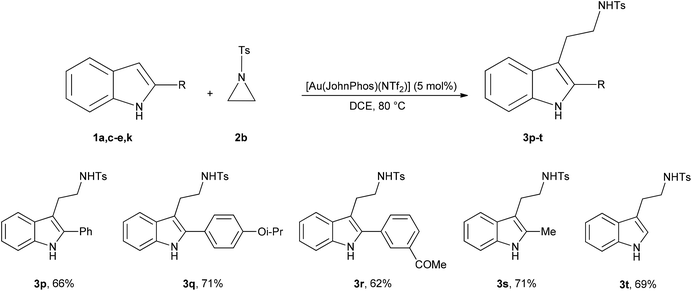
Next, we turned our attention to the aziridine nucleus testing the reactivity of N-tosylaziridine (2b) (Scheme 4).
The reaction works well also with the unsubstituted N-tosylaziridine (2b). Tryptamines 3p–t were obtained in 62–71% yield using a slight excess (1.2 equivalents) of thermally fragile 2b. Working with a larger excess of 2b (1.5 or 2.0 equivalents) resulted in the isolation of dirty reaction products, contaminated by inseparable tarry decomposition compounds arising from 2b.
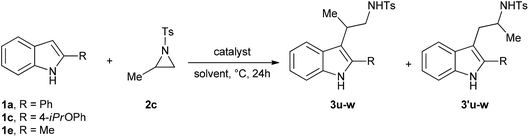
Then, we focused on the ring opening reactions of racemic 2-methyl-N-tosylaziridine (2c) with indoles 1 (Table 2).
| Entrya | Indole | Catalyst (mol%) | Solvent | T, °C | Overall yield, % | Ratio 3/3′ |
|---|---|---|---|---|---|---|
| a Entries 1–3, 5–9: reaction conditions: to a solution of indole (0.3 mmol) and aziridine (1.1 equiv.) in DCE (2 mL, 0.15 M), a catalyst was added and the mixture was stirred for the stated time and temperature. Entry 4: see ref. 3i. Entries 10–13: reaction conditions: to a solution of indole (0.3 mmol) and aziridine (0.15 equiv.) in DCE (2 mL, 0.15 M), a catalyst was added and the mixture was stirred at 80 °C for 24 h. | ||||||
| 1 | 1a | [Au(JohnPhos)(NTf2)] (5 mol%) | DCE | 80 | 98 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 1c | [Au(JohnPhos)(NTf2)] (5 mol%) | DCE | 80 | 86 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 1e | [Au(JohnPhos)(NTf2)] (5 mol%) | DCE | 80 | 92 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 1a | BF3·OEt2 (1.5 equiv.) | DCM | 20 | 55 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 1a | [Au(JohnPhos)(SbF6)(CH3CN)] (5 mol%) | DCE | 80 | 91 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 1a | [Au(PPh3)(NTf2)] (5 mol%) | DCE | 80 | 31 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 1a | [Au(IPr)(NTf2)] (5 mol%) | DCE | 80 | 99 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 1a | [Au(IPr)(SbF6)(CH3CN)] (5 mol%) | DCE | 80 | 96 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 1a | AuCl/AgOTf (5 mol%) | DCE | 80 | 27 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | 1a | AuCl/AgOTf (5 mol%) | DCE | 80 | 46 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | 1a | AuCl/AgNTf2 (5 mol%) | DCE | 80 | 70 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | 1a | AuCl/AgSbF6 (5 mol%) | DCE | 80 | 70 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | 1a | AgOTf (5 mol%) | DCE | 80 | — | — |
Generally, quite unsatisfactory results were obtained working with 2-methyl-N-tosylaziridine 2c. The first three experiments (entries 1–3), performed with indoles 1a,c,e under standard reaction conditions, resulted in the isolation in excellent overall yields of an inseparable mixture of both conceivable regioisomeric tryptamines 3u–w and 3′u–w in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios. As reported in the Introduction, regioselectivity in the ring-opening reaction of activated 2-alkylaziridines, using indoles as nucleophiles, is still a challenging objective and only one example of a regioselective reaction has been reported until now in the presence of an excess (1.5 equiv.) of boron trifluoride etherate.3i Thus, we performed the reaction between 1a and 2c under the conditions reported by Farr and co-workers (entry 4) attaining 3u and 3′u in a 12
1 ratios. As reported in the Introduction, regioselectivity in the ring-opening reaction of activated 2-alkylaziridines, using indoles as nucleophiles, is still a challenging objective and only one example of a regioselective reaction has been reported until now in the presence of an excess (1.5 equiv.) of boron trifluoride etherate.3i Thus, we performed the reaction between 1a and 2c under the conditions reported by Farr and co-workers (entry 4) attaining 3u and 3′u in a 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Next, with the aim to improve the regioisomeric ratios between 3 and 3′ under catalytic conditions, we tested several cationic gold(I) complexes varying both the ligands and the counterions (entries 5–8) achieving quite disappointing results. Better results could be obtained in the presence of simple gold(I) salts such as gold(I) triflate, generated in situ by mixing equimolecular amounts of gold(I) chloride and silver triflate. Thus, the reaction gave rise to compounds 3u and 3′u in a 7
1 ratio. Next, with the aim to improve the regioisomeric ratios between 3 and 3′ under catalytic conditions, we tested several cationic gold(I) complexes varying both the ligands and the counterions (entries 5–8) achieving quite disappointing results. Better results could be obtained in the presence of simple gold(I) salts such as gold(I) triflate, generated in situ by mixing equimolecular amounts of gold(I) chloride and silver triflate. Thus, the reaction gave rise to compounds 3u and 3′u in a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (entry 9). The yield and regioisomeric ratio comparable to those obtained with boron trifluoride could be obtained working in the presence of 2 equivalents of indole 1a (entry 10). A brief screening on the nature of silver salts was then performed revealing that AuCl/AgSbF6 and AuCl/AgNTf2 were the catalysts of choice to achieve 3u and 3′u in 70% yield and 7
1 ratio (entry 9). The yield and regioisomeric ratio comparable to those obtained with boron trifluoride could be obtained working in the presence of 2 equivalents of indole 1a (entry 10). A brief screening on the nature of silver salts was then performed revealing that AuCl/AgSbF6 and AuCl/AgNTf2 were the catalysts of choice to achieve 3u and 3′u in 70% yield and 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (entries 11 and 12). A control experiment performed with silver triflate as the catalyst failed to give the desired compound (entry 13). Structures and ratios between the two regioisomers were assigned via NMR analysis.13 A control reaction between indole 1a and aziridine (S)2c under the reaction conditions reported in Table 2, entry 12 resulted in the isolation of a mixture of (R)3u (94% ee) and 3′u in 68% overall yield (10
1 ratio (entries 11 and 12). A control experiment performed with silver triflate as the catalyst failed to give the desired compound (entry 13). Structures and ratios between the two regioisomers were assigned via NMR analysis.13 A control reaction between indole 1a and aziridine (S)2c under the reaction conditions reported in Table 2, entry 12 resulted in the isolation of a mixture of (R)3u (94% ee) and 3′u in 68% overall yield (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) (Scheme 5).
1 ratio) (Scheme 5).
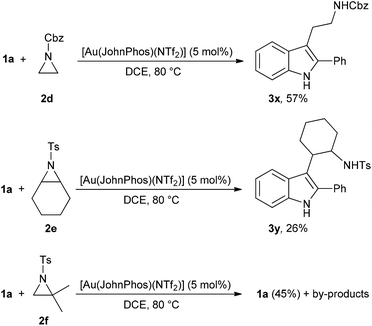
Moreover, several additional experiments were performed with indole 1a and aziridines 2d–f (Scheme 6).
Under standard reaction conditions indole 1a reacts with aziridines 2d and 2e giving rise to tryptamines 3x,y in low to moderate yields, besides unreacted 1a. Moreover, the same reaction performed in the presence of aziridine 2f resulted in the isolation of unreacted 1a (45%) alongside a mixture of unidentified compounds.
Finally, we want to report our preliminary results on the dearomative domino addition/annulation reactions between N,3-dimethylindole 1p, N-benzyl-3-methylindole 1q, 2,3-dimethylindole 1r and 3-allyl-N-methylindole 1s with aziridines 2a and 2b, under cationic [Au(JohnPhos)(NTf2)] gold(I) catalysis (Scheme 7).
Excellent results on related dearomative reactions, involving unsubstituted aziridines,14 symmetrically C2/C3 substituted aziridines15 or C2 substituted aziridines,16 have been recently reported.
Under cationic gold(I) catalysis, the dearomatization reactions of indoles 1p–r with aziridine 2a proceeded in moderate yields giving rise to the corresponding diastereoisomeric dihydropyrroloindolines (±)4a–c and (±)4′a–c as racemic mixtures. Diastereoisomers (±)4a/(±)4′a and (±)4c/(±)4′c could be separated by column chromatography and the structures were established by comparison with the reported data16 for (±)4a and (±)4′a and by 2D NOESY experiments for (±)4c and (±)4′c. Diastereoisomers (±)4b/(±)4′b were characterized as a mixture via NMR and by comparison with the reported data.16 Reactions with indoles 1p,q were repeated also in the presence of chiral aziridine (R)2a giving rise to enantiomeric enriched diastereoisomers 4a (96% ee), 4′a (96% ee)/4b (99% ee) and 4′b (99% ee). 4a/4′a and 4b/4′b were evaluated as mixtures via chiral HPLC analysis, after chromatographic purification.13,17
Working with indole 1p and aziridine 2b, the indoline 4d was obtained in 55% yield after optimization of the reaction conditions. In particular, the reactions performed under standard conditions (ratio indole/aziridine 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2, 0.15 M solution in dichloroethane) at 80 and at 120 °C under conventional heating or at temperatures ranging from 120 to 150 °C under microwave irradiation gave unsatisfactory results (yields 18–39%, Scheme 7, entries 1–4). A brief optimization study on the reagent ratios and reaction concentrations revealed that doubling the equivalents of 2b has no effect on the product yield (Scheme 7, entry 5). On the other hand, working with two equivalents of indole 1p, at 0.3 M and 0.6 M concentrations, the desired compound could be isolated in 45% and 55% yields, respectively (Scheme 7, entries 6 and 7). Under optimized reaction conditions, 3-allyl-N-methylindole (1s) was tested in the dearomative reaction giving rise to indoline 4e in 45% yield.
1.2, 0.15 M solution in dichloroethane) at 80 and at 120 °C under conventional heating or at temperatures ranging from 120 to 150 °C under microwave irradiation gave unsatisfactory results (yields 18–39%, Scheme 7, entries 1–4). A brief optimization study on the reagent ratios and reaction concentrations revealed that doubling the equivalents of 2b has no effect on the product yield (Scheme 7, entry 5). On the other hand, working with two equivalents of indole 1p, at 0.3 M and 0.6 M concentrations, the desired compound could be isolated in 45% and 55% yields, respectively (Scheme 7, entries 6 and 7). Under optimized reaction conditions, 3-allyl-N-methylindole (1s) was tested in the dearomative reaction giving rise to indoline 4e in 45% yield.
Discussion
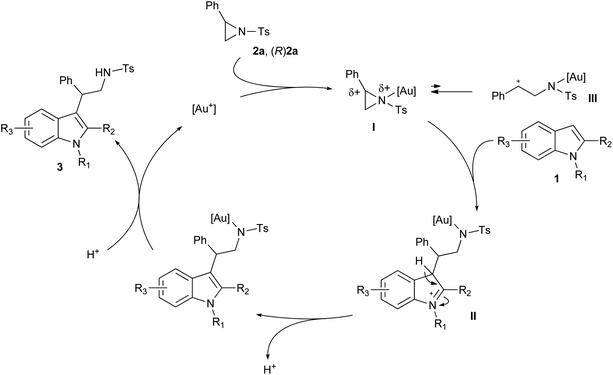
As a general remark it can be stated that cationic gold(I) complexes are active catalysts in the ring-opening reactions of activated aziridine with indoles as nucleophiles. In particular, [Au(JohnPhos)NTf2] is the catalyst of choice for the ring-opening reactions performed with 2-phenyl-N-tosylaziridine 2a. Thus, the nucleophilic attack of indoles 1a–o, through the C3 carbon atom, occurs regioselectively at the C2 of the aziridine ring, yielding the corresponding tryptamine derivatives 3a–o in excellent yields with a catalyst loading of only 5 mol%. Moreover, the ring-opening reactions provide racemic mixtures when performed with achiral aziridine 2a and is stereospecific employing the chiral aziridine (R)2a as the starting material. Based on these experimental results and literature reports, a reaction mechanism can be postulated for the ring-opening reactions of 2a and (R)2a with indoles 1 (Scheme 8). Therefore, azaphilic10,18 activation of aziridine by a cationic gold(I) catalyst produces an activated complex (I). Then indole 1 attacks regioselectively at the benzylic carbon of I producing a new intermediate IIvia a Friedel–Crafts-type reaction. Rearomatization and protodeauration steps conclude the catalytic cycle affording 3 and restoring the catalyst.The stereospecificity attained with (R)2a accounts for the intermediacy of a strongly polarized species like I, reacting with 1via the SN2-type mechanism. Besides, the intermediacy of a zwitterionic species III, Scheme 8, can be disregarded as it would provide racemic 3 by SN1-type mechanism. Unfortunately, the same regioselectivity was not observed in the ring-opening reactions involving 2-methyl-N-tosylaziridine (2b) under [Au(JohnPhos)NTf2] catalysis. As reported in the literature and confirmed by our experiments (see Table 2), boron trifluoride is able to almost regioselectively address the nucleophilic attack at the more hindered carbon atom of the aziridine ring. It seems that an excess of a hard Lewis acid like boron trifluoride is essential to establish a strong interaction within both reacting substrates and to trigger the reaction toward the C2 selective ring-opening reaction path.3i,19 Instead, cationic gold(I) complexes in a catalytic amount weakly interact with the aziridine nitrogen, and in the absence of a strong directing group like a phenyl ring in aziridine 2a, the reaction outcomes are driven by steric and electronic issues making possible two opposite reaction paths. Both of them involve the ring-opening reaction of aziridine 2b, respectively, at C2 and C3, affording mixtures of both conceivable tryptamines 3 and 3′. In line with these observations, it is worth noting that using 5 mol% of more electrophilic naked cationic gold salts improves the regioisomeric ratio between 3u and 3′u. Importantly, the [Au(JohnPhos)NTf2] catalyst proves effective also in the dearomative reactions of C3 substituted indoles. In this latter case, a reaction intermediate analogous to II, Scheme 8, evolves by intramolecular amination reaction and regeneration of the catalyst.
Conclusions
In this work we established the high efficiency of a cationic gold(I) catalyst [Au(JohnPhos)(NTf2)] in the ring opening reactions of 2-phenyl-N-tosylaziridine 2a and (R)2a with indoles as nucleophiles. In particular, we gathered a large collection of tryptamine derivatives in high yields, with complete stereochemical control and at a catalyst loading of only 5 mol%. To the best of our knowledge, only sporadic examples of related Lewis acid catalyzed reactions have been reported until now.3h,a,i Furthermore, it should be pointed out that, depending on the nature of the Lewis acid employed, previously reported reactions could suffer from lack of stereocontrol due to racemization of the starting aziridine during the reaction course.16a The results obtained with 2a, besides those obtained with N-tosylaziridine itself (2b), represent the key strength of our work. In contrast, less exciting results have been achieved with 2-methyl-N-tosylaziridine (2c) and in the dearomative domino addition/annulation reactions. In the first case, we observed a lack of regiochemical control even if nearly quantitative reaction yields could be obtained. Dearomative reactions giving rise to pyrroloindoline derivatives, besides excellent stereocontrol, present several drawbacks. Moderate yields and low diastereoselection are the main weak points that must be addressed and further efforts in these directions are ongoing in our laboratory. Also the results reported for aziridines 2d–f in Scheme 6 could represent a good starting point for further investigations.Experimental
General experimental details
All chemicals and solvents are commercially available and were used after distillation or treatment with drying agents. Silica gel F254 thin-layer plates were employed for thin-layer chromatography (TLC). Silica gel 40–63 micron per 60 Å was employed for flash column chromatography. Melting points were measured with a Perkin-Elmer DSC 6 calorimeter at a heating rate of 5 °C min−1 and are uncorrected. 1H and 13C-NMR spectra were determined with a Varian-Gemini 200, a Bruker 300 or 500 Avance spectrometer at room temperature in CDCl3, CD2Cl2 or d6-acetone with residual solvent peaks as the internal reference. The APT sequence was used to distinguish the methine and methyl carbon signals from those arising from methylene and quaternary carbon atoms. Two-dimensional NMR experiments were performed, where appropriate, to aid the assignment of structures. Low-resolution MS spectra were recorded with a Thermo-Finnigan LCQ advantage AP electrospray/ion trap equipped instrument using a syringe pump device to directly inject sample solutions. Microwave promoted reactions were performed with a single-mode Personal Chemistry microwave synthesizer “Emrys Creator,” using sealed glass vessels. The temperature was detected with an infrared sensor. Indoles 1a, 1e, 1k, 1n, 1o, 1p, 1r are commercially available and were used without any further purification. Indoles 1b–d, and 1l are known compounds and were prepared as reported in the literature.9,20 Indoles 1q and 1s are known compounds and were prepared according to literature procedures.21 Indole 1h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 and indoles 1i and 1j
22 and indoles 1i and 1j![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 were prepared according to the procedures reported in ref. 9 and 20 (see below). Indoles 1f and 1g were prepared as reported in ref. 24 and 8b, respectively.24,8b Indole 1m is a new compound and was prepared as reported below. Aziridines 2a–f are known compounds and were prepared according to standard procedures.25 AuCl3, AuCl[Au(PPh3)Cl], [Au(JohnPhos)Cl], [Au(IPr)Cl], AgNTf2, AgOTf, In(OTf)3, Zn(NTf)2 and TfOH were purchased from commercial suppliers and used as received, the rest of the gold catalysts were prepared according to literature procedures.26
23 were prepared according to the procedures reported in ref. 9 and 20 (see below). Indoles 1f and 1g were prepared as reported in ref. 24 and 8b, respectively.24,8b Indole 1m is a new compound and was prepared as reported below. Aziridines 2a–f are known compounds and were prepared according to standard procedures.25 AuCl3, AuCl[Au(PPh3)Cl], [Au(JohnPhos)Cl], [Au(IPr)Cl], AgNTf2, AgOTf, In(OTf)3, Zn(NTf)2 and TfOH were purchased from commercial suppliers and used as received, the rest of the gold catalysts were prepared according to literature procedures.26
Preparation and characterization data for compounds 1h–j,m
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (50 mL). The two phases were separated and the aqueous layer was extracted with EtOAc (2 × 15 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated in vacuum to yield 2-(phenylethynyl)-1H-indole (1h) (0.59 g, 94%) as a yellow solid. 1H-NMR (200 MHz, CD2Cl2): δ 8.39 (s, 1H), 7.59 (m, 3H), 7.40 (m, 4H), 7.27 (t, J = 6.9 Hz, 1H), 7.15 (t, J = 7.3 Hz, 1H), 6.87 (s, 1H). 13C-NMR (50 MHz, CD2Cl2): δ 136.5 (C), 131.6 (2 × CH), 128.9 (CH), 128.7 (2 × CH), 128.0 (C), 123.8 (CH), 122.8 (C), 120.9(CH), 120.7 (CH), 119.0 (C), 111.0 (CH), 108.8 (CH), 92.6 (C), 81.8 (C). ESI-MSm/z 218 (M + H+, 100). Data are in agreement with those reported in ref. 22.
1 (50 mL). The two phases were separated and the aqueous layer was extracted with EtOAc (2 × 15 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated in vacuum to yield 2-(phenylethynyl)-1H-indole (1h) (0.59 g, 94%) as a yellow solid. 1H-NMR (200 MHz, CD2Cl2): δ 8.39 (s, 1H), 7.59 (m, 3H), 7.40 (m, 4H), 7.27 (t, J = 6.9 Hz, 1H), 7.15 (t, J = 7.3 Hz, 1H), 6.87 (s, 1H). 13C-NMR (50 MHz, CD2Cl2): δ 136.5 (C), 131.6 (2 × CH), 128.9 (CH), 128.7 (2 × CH), 128.0 (C), 123.8 (CH), 122.8 (C), 120.9(CH), 120.7 (CH), 119.0 (C), 111.0 (CH), 108.8 (CH), 92.6 (C), 81.8 (C). ESI-MSm/z 218 (M + H+, 100). Data are in agreement with those reported in ref. 22.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded ethyl 2-(pent-1-yn-1-yl)-1H-indole-1-carboxylate (0.72 g, 95%) as a yellow oil. 1H-NMR (200 MHz, CDCl3): δ 8.13 (d, J = 8.2 Hz, 1H), 7.48 (m, 1H), 7.37–7.17 (m, 2H), 6.82 (s, 1H), 4.51 (q, J = 7.1 Hz, 2H), 2.48 (t, J = 7.1 Hz, 2H), 1.68 (sextet, J = 7.1 Hz, 2H), 1.49 (t, J = 7.1 Hz, 3H), 1.08 (t, J = 7.3 Hz, 3H). Ethyl 2-(pent-1-yn-1-yl)-1H-indole-1-carboxylate (0.72 g, 2.82 mmol) was dissolved in MeOH (19 mL). Solid K2CO3 (0.39 g, 2.82 mmol) was added and the mixture was stirred for 2 h at 40 °C. After that time, the solvent was removed in vacuum and the residue was dissolved in H2O/EtOAc 1
2) yielded ethyl 2-(pent-1-yn-1-yl)-1H-indole-1-carboxylate (0.72 g, 95%) as a yellow oil. 1H-NMR (200 MHz, CDCl3): δ 8.13 (d, J = 8.2 Hz, 1H), 7.48 (m, 1H), 7.37–7.17 (m, 2H), 6.82 (s, 1H), 4.51 (q, J = 7.1 Hz, 2H), 2.48 (t, J = 7.1 Hz, 2H), 1.68 (sextet, J = 7.1 Hz, 2H), 1.49 (t, J = 7.1 Hz, 3H), 1.08 (t, J = 7.3 Hz, 3H). Ethyl 2-(pent-1-yn-1-yl)-1H-indole-1-carboxylate (0.72 g, 2.82 mmol) was dissolved in MeOH (19 mL). Solid K2CO3 (0.39 g, 2.82 mmol) was added and the mixture was stirred for 2 h at 40 °C. After that time, the solvent was removed in vacuum and the residue was dissolved in H2O/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (50 mL). The two phases were separated and the aqueous layer was extracted with EtOAc (2 × 15 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated in vacuum to yield 2-(pent-1-yn-1-yl)-1H-indole (1i) (0.49 g, 94%) as a brown wax. 1H-NMR (200 MHz, CDCl3): δ 8.09 (s, 1H), 7.56 (m, 1H), 7.35–7.02 (m, 3H), 6.66 (d, J = 1.3 Hz, 1H), 2.44 (t, J = 7.0 Hz, 2H), 1.63 (sextet, J = 7.1 Hz, 2H), 1.07 (t, J = 7.3 Hz, 3H). 13C-NMR (50 MHz, CDCl3): δ 136.0 (C), 128.1 (C), 123.2 (CH), 120.8 (CH), 120.5 (CH), 119.8 (C), 110.8 (CH), 107.7 (CH), 93.9 (C), 73.4 (C), 22.3 (CH2), 21.8 (CH2), 13.8 (CH3). ESI-MSm/z 182 (M − H+, 100).
1 (50 mL). The two phases were separated and the aqueous layer was extracted with EtOAc (2 × 15 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated in vacuum to yield 2-(pent-1-yn-1-yl)-1H-indole (1i) (0.49 g, 94%) as a brown wax. 1H-NMR (200 MHz, CDCl3): δ 8.09 (s, 1H), 7.56 (m, 1H), 7.35–7.02 (m, 3H), 6.66 (d, J = 1.3 Hz, 1H), 2.44 (t, J = 7.0 Hz, 2H), 1.63 (sextet, J = 7.1 Hz, 2H), 1.07 (t, J = 7.3 Hz, 3H). 13C-NMR (50 MHz, CDCl3): δ 136.0 (C), 128.1 (C), 123.2 (CH), 120.8 (CH), 120.5 (CH), 119.8 (C), 110.8 (CH), 107.7 (CH), 93.9 (C), 73.4 (C), 22.3 (CH2), 21.8 (CH2), 13.8 (CH3). ESI-MSm/z 182 (M − H+, 100).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (15 mL). The two phases were separated and the aqueous layer was extracted with EtOAc (2 × 5 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated in a vacuum to yield 2-allyl-1H-indole (1j) (0.11 g, 60%) as a white solid. 1H-NMR (200 MHz, CDCl3): δ 7.89 (s, 1H), 7.54 (d, J = 6.8 Hz, 1H), 7.36–7.00 (m, 3H), 6.28 (s, 1H), 6.00 (m, 1H), 5.21 (m, 2H), 3.55 (d, J = 6.3 Hz, 2H). Data are in agreement with those reported in ref. 23.
1 (15 mL). The two phases were separated and the aqueous layer was extracted with EtOAc (2 × 5 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated in a vacuum to yield 2-allyl-1H-indole (1j) (0.11 g, 60%) as a white solid. 1H-NMR (200 MHz, CDCl3): δ 7.89 (s, 1H), 7.54 (d, J = 6.8 Hz, 1H), 7.36–7.00 (m, 3H), 6.28 (s, 1H), 6.00 (m, 1H), 5.21 (m, 2H), 3.55 (d, J = 6.3 Hz, 2H). Data are in agreement with those reported in ref. 23.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to afford N,N-diethyl-1H-indol-6-amine (1m) (95 mg, 83%) as pale brown oil. 1H-NMR (200 MHz, CDCl3): δ 7.87 (s, 1H), 7.47 (d, J = 9.1 Hz, 1H), 7.01 (m, 1H), 6.78 (m, 2H), 6.42 (m, 1H), 3.36 (q, J = 7.0 Hz, 4H), 1.16 (t, J = 7.0 Hz, 6H). 13C-NMR (C6D6, 50 MHz): δ 145.2 (C), 138.0 (C), 121.7 (CH), 121.3 (CH), 120.9 (C), 110.7 (CH), 102.4 (CH), 96.3 (CH), 45.7 (2 × CH2), 12.7 (2 × CH3). ESI-MSm/z 189 (M + H+, 100).
3) to afford N,N-diethyl-1H-indol-6-amine (1m) (95 mg, 83%) as pale brown oil. 1H-NMR (200 MHz, CDCl3): δ 7.87 (s, 1H), 7.47 (d, J = 9.1 Hz, 1H), 7.01 (m, 1H), 6.78 (m, 2H), 6.42 (m, 1H), 3.36 (q, J = 7.0 Hz, 4H), 1.16 (t, J = 7.0 Hz, 6H). 13C-NMR (C6D6, 50 MHz): δ 145.2 (C), 138.0 (C), 121.7 (CH), 121.3 (CH), 120.9 (C), 110.7 (CH), 102.4 (CH), 96.3 (CH), 45.7 (2 × CH2), 12.7 (2 × CH3). ESI-MSm/z 189 (M + H+, 100).
Preparation and characterization data for compounds 3a–t, (S)3a, (S)3c–e, (S)3k, 3x,y
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded 3a (137 mg, 98%) as a white solid (m.p.: 165–167 °C). 1H NMR (300 MHz, CDCl3): δ 8.27 (s, 1H), 7.54–7.38 (m, 8H), 7.32–7.09 (m, 9H), 6.97 (t, J = 7.5 Hz, 1H), 4.49 (dd, J = 10.7, 6.1 Hz, 1H), 4.24 (dd, J = 9.1, 2.8 Hz, 1H), 3.84–3.62 (m, 2H), 2.42 (s, 3H). 1H NMR (300 MHz, CDCl3 + D2O): δ 7.54–7.38 (m, 8H), 7.32–7.09 (m, 9H), 6.97 (t, J = 7.5 Hz, 1H), 4.49 (dd, J = 10.7, 6.1 Hz, 1H), 3.80 (dd, J = 12.2, 6.1 Hz, 1H), 3.70 (dd, J = 10.9, 12.0 Hz, 1H), 2.41 (s, 3H). 13C NMR (50 MHz, CDCl3): δ 143.4 (C), 141.9 (C), 138.0 (C), 136.5 (C), 136.4 (C), 132.4 (C), 129.8 (2 × CH), 129.1 (2 × CH), 128.9 (2 × CH), 128.8 (2 × CH), 128.6 (CH), 127.9 (2 × CH), 127.3 (C), 127.2 (2 × CH), 126.9 (CH), 122.7 (CH), 120.4 (CH), 120.3 (CH), 111.8 (CH), 109.7 (C), 46.9 (CH2), 42.5 (CH), 21.8 (CH3). ESI-MSm/z 465 (M − H+, 100). Calcd for C29H26N2O2S [466.59]: C 74.65, H 5.62, N 6.00; found C 74.38, H 5.78, N 5.86.
2) yielded 3a (137 mg, 98%) as a white solid (m.p.: 165–167 °C). 1H NMR (300 MHz, CDCl3): δ 8.27 (s, 1H), 7.54–7.38 (m, 8H), 7.32–7.09 (m, 9H), 6.97 (t, J = 7.5 Hz, 1H), 4.49 (dd, J = 10.7, 6.1 Hz, 1H), 4.24 (dd, J = 9.1, 2.8 Hz, 1H), 3.84–3.62 (m, 2H), 2.42 (s, 3H). 1H NMR (300 MHz, CDCl3 + D2O): δ 7.54–7.38 (m, 8H), 7.32–7.09 (m, 9H), 6.97 (t, J = 7.5 Hz, 1H), 4.49 (dd, J = 10.7, 6.1 Hz, 1H), 3.80 (dd, J = 12.2, 6.1 Hz, 1H), 3.70 (dd, J = 10.9, 12.0 Hz, 1H), 2.41 (s, 3H). 13C NMR (50 MHz, CDCl3): δ 143.4 (C), 141.9 (C), 138.0 (C), 136.5 (C), 136.4 (C), 132.4 (C), 129.8 (2 × CH), 129.1 (2 × CH), 128.9 (2 × CH), 128.8 (2 × CH), 128.6 (CH), 127.9 (2 × CH), 127.3 (C), 127.2 (2 × CH), 126.9 (CH), 122.7 (CH), 120.4 (CH), 120.3 (CH), 111.8 (CH), 109.7 (C), 46.9 (CH2), 42.5 (CH), 21.8 (CH3). ESI-MSm/z 465 (M − H+, 100). Calcd for C29H26N2O2S [466.59]: C 74.65, H 5.62, N 6.00; found C 74.38, H 5.78, N 5.86.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded 3b (137 mg, 95%) as a white thick oil. 1H-NMR (300 MHz, CDCl3): δ 7.55–7.12 (m, 17H), 6.95 (t, J = 7.0 Hz, 1H), 4.25 (dd, J = 10.6, 6.6 Hz, 1H), 3.72–3.51 (m, 5H), 2.44 (s, 3H). 13C-NMR (75 MHz, CDCl3): δ 143.6 (C), 142.2 (C), 140.9 (C), 137.8 (C), 136.6 (C) 131.5 (C), 131.2 (2 × CH), 130.0 (2 × CH), 129.0 (CH), 128.9 (2 × CH), 128.8 (2 × CH), 128.0 (2 × CH), 127.5 (2 × CH), 126.9 (CH), 126.2 (C), 122.3 (CH), 120.2 (CH), 120.1 (CH), 110.3 (C), 110.2 (CH), 46.9 (CH2), 42.8 (CH), 31.3 (CH3), 21.9 (CH3). ESI-MSm/z 503 [M + Na+, 100]. Calcd for C30H28N2O2S [480.63]: C 74.97, H 5.87, N 5.83; found C 75.29, H 5.71, N 5.97.
2) yielded 3b (137 mg, 95%) as a white thick oil. 1H-NMR (300 MHz, CDCl3): δ 7.55–7.12 (m, 17H), 6.95 (t, J = 7.0 Hz, 1H), 4.25 (dd, J = 10.6, 6.6 Hz, 1H), 3.72–3.51 (m, 5H), 2.44 (s, 3H). 13C-NMR (75 MHz, CDCl3): δ 143.6 (C), 142.2 (C), 140.9 (C), 137.8 (C), 136.6 (C) 131.5 (C), 131.2 (2 × CH), 130.0 (2 × CH), 129.0 (CH), 128.9 (2 × CH), 128.8 (2 × CH), 128.0 (2 × CH), 127.5 (2 × CH), 126.9 (CH), 126.2 (C), 122.3 (CH), 120.2 (CH), 120.1 (CH), 110.3 (C), 110.2 (CH), 46.9 (CH2), 42.8 (CH), 31.3 (CH3), 21.9 (CH3). ESI-MSm/z 503 [M + Na+, 100]. Calcd for C30H28N2O2S [480.63]: C 74.97, H 5.87, N 5.83; found C 75.29, H 5.71, N 5.97.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded 3c (142 mg, 90%) as a white solid (m.p.: 75–78 °C). 1H-NMR (200 MHz, CDCl3): δ 8.18 (s, 1H), 7.50–7.36 (m, 3H), 7.32–7.08 (m, 11H), 6.97–6.85 (m, 3H), 4.59 (hept, J = 6.0 Hz, 1H), 4.45 (dd, J = 10.7, 6.2 Hz, 1H), 4.22 (dd, J = 9.0, 2.9 Hz, 1H), 3.84–3.55 (m, 2H), 2.40 (s, 3H), 1.39 (d, J = 6.2 Hz, 3H), 1.37 (d, J = 6.0 Hz, 3H). 13C-NMR (50 MHz, CDCl3): δ 158.5 (C), 143.3 (C), 142.0 (C), 138.0 (C), 136.5 (C), 136.3 (C), 130.1 (2 × CH), 129.8 (2 × CH), 128.8 (2 × CH), 127.9 (2 × CH), 127.4 (C), 127.2 (2 × CH), 126.9 (CH), 124.4 (C), 122.4 (CH), 120.3 (CH), 120.1 (CH), 116.3 (2 × CH), 111.4 (CH), 109.1 (C), 70.3 (CH), 46.8 (CH2), 42.4 (CH), 22.3 (CH3), 22.2 (CH3), 21.7 (CH3). ESI-MSm/z 523 [M − H+, 100]. Calcd for C32H32N2O3S [524.67]: C 73.25, H 6.15, N 5.34; found: C 73.02, H 6.27, N 5.49.
2) yielded 3c (142 mg, 90%) as a white solid (m.p.: 75–78 °C). 1H-NMR (200 MHz, CDCl3): δ 8.18 (s, 1H), 7.50–7.36 (m, 3H), 7.32–7.08 (m, 11H), 6.97–6.85 (m, 3H), 4.59 (hept, J = 6.0 Hz, 1H), 4.45 (dd, J = 10.7, 6.2 Hz, 1H), 4.22 (dd, J = 9.0, 2.9 Hz, 1H), 3.84–3.55 (m, 2H), 2.40 (s, 3H), 1.39 (d, J = 6.2 Hz, 3H), 1.37 (d, J = 6.0 Hz, 3H). 13C-NMR (50 MHz, CDCl3): δ 158.5 (C), 143.3 (C), 142.0 (C), 138.0 (C), 136.5 (C), 136.3 (C), 130.1 (2 × CH), 129.8 (2 × CH), 128.8 (2 × CH), 127.9 (2 × CH), 127.4 (C), 127.2 (2 × CH), 126.9 (CH), 124.4 (C), 122.4 (CH), 120.3 (CH), 120.1 (CH), 116.3 (2 × CH), 111.4 (CH), 109.1 (C), 70.3 (CH), 46.8 (CH2), 42.4 (CH), 22.3 (CH3), 22.2 (CH3), 21.7 (CH3). ESI-MSm/z 523 [M − H+, 100]. Calcd for C32H32N2O3S [524.67]: C 73.25, H 6.15, N 5.34; found: C 73.02, H 6.27, N 5.49.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) yielded 3d (148 mg, 97%) as a yellowish solid (m.p.: 77–79 °C). 1H-NMR (200 MHz, CDCl3): δ 8.92 (s, 1H), 7.89 (m, 2H), 7.64–7.05 (m, 14H), 6.96 (t, J = 7.2, 1H), 4.48 (m, 2H), 3.75 (m, 2H), 2.42 (s, 3H), 2.37 (s, 3H). 13C NMR (50 MHz, CDCl3): δ 198.2 (C), 143.5 (C), 141.8 (C), 137.7 (C), 136.8 (C), 136.7 (C), 136.6 (C), 133.3 (CH), 133.0 (C), 129.8 (2 × CH), 129.3 (CH), 128.9 (2 × CH), 128.9 (CH), 128.0 (2 × CH), 127.3 (C), 127.2 (2 × CH), 127.1 (CH), 122.9 (CH), 120.5 (CH), 120.3 (CH), 112.0 (CH), 110.4 (C), 47.1 (CH2), 43.0 (CH), 26.8 (CH3), 21.7 (CH3). 1 CHar is overlapping probably with 2 × CH at 128.0. ESI-MSm/z 507 [M − H+, 100]. Calcd for C31H28N2O3S [508.63]: C 73.20, H 5.55, N 5.51; found: C 73.58, H 5.41, N 5.62.
3) yielded 3d (148 mg, 97%) as a yellowish solid (m.p.: 77–79 °C). 1H-NMR (200 MHz, CDCl3): δ 8.92 (s, 1H), 7.89 (m, 2H), 7.64–7.05 (m, 14H), 6.96 (t, J = 7.2, 1H), 4.48 (m, 2H), 3.75 (m, 2H), 2.42 (s, 3H), 2.37 (s, 3H). 13C NMR (50 MHz, CDCl3): δ 198.2 (C), 143.5 (C), 141.8 (C), 137.7 (C), 136.8 (C), 136.7 (C), 136.6 (C), 133.3 (CH), 133.0 (C), 129.8 (2 × CH), 129.3 (CH), 128.9 (2 × CH), 128.9 (CH), 128.0 (2 × CH), 127.3 (C), 127.2 (2 × CH), 127.1 (CH), 122.9 (CH), 120.5 (CH), 120.3 (CH), 112.0 (CH), 110.4 (C), 47.1 (CH2), 43.0 (CH), 26.8 (CH3), 21.7 (CH3). 1 CHar is overlapping probably with 2 × CH at 128.0. ESI-MSm/z 507 [M − H+, 100]. Calcd for C31H28N2O3S [508.63]: C 73.20, H 5.55, N 5.51; found: C 73.58, H 5.41, N 5.62.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded 3e (112 mg, 92%) as a white solid (m.p.: 147–150 °C). 1H-NMR (200 MHz, CDCl3): δ 7.97 (s, 1H), 7.62 (d, J = 8.3 Hz, 2H), 7.31–7.08 (m, 9H), 6.90 (t, J = 7.4 Hz, 1H), 4.41 (dd, J = 10.4, 6.1 Hz, 1H), 4.33 (dd, J = 8.9, 2.9 Hz, 1H), 3.84 (ddd, J = 12.1, 9.1, 6.2, 1H), 3.61 (ddd, J = 12.1, 10.6, 3.2, 1H), 2.45 (s, 3H), 2.33 (s, 3H). 1H-NMR (200 MHz, CDCl3 + D2O): δ 7.62 (d, J = 8.3 Hz, 2H), 7.31–7.08 (m, 9H), 6.90 (t, J = 7.4 Hz, 1H), 4.41 (dd, J = 10.5, 6.1 Hz, 1H), 4.33 (dd, J = 8.9, 2.9 Hz, 1H), 3.83 (dd, J = 12.1, 6.1, 1H), 3.60 (dd, J = 12.1, 10.6, 1H), 2.45 (s, 3H), 2.32 (s, 3H). 13C NMR (50 MHz, CDCl3): δ 143.7 (C), 141.7 (C), 136.7 (C), 135.8 (C), 133.9 (C), 130.0 (2 × CH), 128.8 (2 × CH), 127.9 (2 × CH), 127.3 (2 × CH), 127.2 (C), 126.8 (CH), 121.4 (CH), 119.8 (CH), 118.9 (CH), 111.1 (CH), 109.2 (C), 46.6 (CH2), 42.3 (CH), 21.8 (CH3), 12.2 (CH3). ESI-MSm/z 403 [M − H+, 100]. Calcd for C24H24N2O2S [404.52]: C 71.26, H 5.98, N 6.93; found: C 71.48, H 6.02, N 6.80.
2) yielded 3e (112 mg, 92%) as a white solid (m.p.: 147–150 °C). 1H-NMR (200 MHz, CDCl3): δ 7.97 (s, 1H), 7.62 (d, J = 8.3 Hz, 2H), 7.31–7.08 (m, 9H), 6.90 (t, J = 7.4 Hz, 1H), 4.41 (dd, J = 10.4, 6.1 Hz, 1H), 4.33 (dd, J = 8.9, 2.9 Hz, 1H), 3.84 (ddd, J = 12.1, 9.1, 6.2, 1H), 3.61 (ddd, J = 12.1, 10.6, 3.2, 1H), 2.45 (s, 3H), 2.33 (s, 3H). 1H-NMR (200 MHz, CDCl3 + D2O): δ 7.62 (d, J = 8.3 Hz, 2H), 7.31–7.08 (m, 9H), 6.90 (t, J = 7.4 Hz, 1H), 4.41 (dd, J = 10.5, 6.1 Hz, 1H), 4.33 (dd, J = 8.9, 2.9 Hz, 1H), 3.83 (dd, J = 12.1, 6.1, 1H), 3.60 (dd, J = 12.1, 10.6, 1H), 2.45 (s, 3H), 2.32 (s, 3H). 13C NMR (50 MHz, CDCl3): δ 143.7 (C), 141.7 (C), 136.7 (C), 135.8 (C), 133.9 (C), 130.0 (2 × CH), 128.8 (2 × CH), 127.9 (2 × CH), 127.3 (2 × CH), 127.2 (C), 126.8 (CH), 121.4 (CH), 119.8 (CH), 118.9 (CH), 111.1 (CH), 109.2 (C), 46.6 (CH2), 42.3 (CH), 21.8 (CH3), 12.2 (CH3). ESI-MSm/z 403 [M − H+, 100]. Calcd for C24H24N2O2S [404.52]: C 71.26, H 5.98, N 6.93; found: C 71.48, H 6.02, N 6.80.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) yielded 3f (112 mg, 90%) as a white solid (m.p.: 130–132 °C). 1H-NMR (200 MHz, CDCl3): δ 8.23 (s, 1H), 7.60 (d, J = 8.3 Hz, 2H), 7.32–7.14 (m, 10H), 6.88 (ddd, J = 8.2, 6.9, 1.1 Hz, 1H), 6.69 (dd, J = 17.5, 11.3 Hz, 1H), 5.52 (d, J = 17.6 Hz, 1H), 5.28 (d, J = 11.3 Hz, 1H), 4.49 (dd, J = 10.3, 6.1 Hz, 1H), 4.30 (dd, J = 8.7, 3.4 Hz, 1H), 3.87 (m, 1H), 3.62 (m, 1H), 2.43 (s, 3H). 13C-NMR (50 MHz, CDCl3): δ 143.6 (C), 141.1 (C), 136.9 (C), 136.7 (C), 134.3 (C), 129.9 (2 × CH), 128.8 (2 × CH), 127.8 (2 × CH), 127.3 (2 × CH), 127.2 (C), 126.9 (CH), 125.3 (CH), 123.4 (CH), 120.3 (CH), 120.1 (CH), 113.6 (CH2), 113.0 (C), 111.3 (CH), 46.5 (CH2), 41.8 (CH), 21.8 (CH3). ESI-MSm/z 439 [M + Na+, 100]. Calcd for C25H24N2O2S [416.54]: C 72.09, H 5.81, N 6.73; C 72.37, H 5.88, N 6.72.
1) yielded 3f (112 mg, 90%) as a white solid (m.p.: 130–132 °C). 1H-NMR (200 MHz, CDCl3): δ 8.23 (s, 1H), 7.60 (d, J = 8.3 Hz, 2H), 7.32–7.14 (m, 10H), 6.88 (ddd, J = 8.2, 6.9, 1.1 Hz, 1H), 6.69 (dd, J = 17.5, 11.3 Hz, 1H), 5.52 (d, J = 17.6 Hz, 1H), 5.28 (d, J = 11.3 Hz, 1H), 4.49 (dd, J = 10.3, 6.1 Hz, 1H), 4.30 (dd, J = 8.7, 3.4 Hz, 1H), 3.87 (m, 1H), 3.62 (m, 1H), 2.43 (s, 3H). 13C-NMR (50 MHz, CDCl3): δ 143.6 (C), 141.1 (C), 136.9 (C), 136.7 (C), 134.3 (C), 129.9 (2 × CH), 128.8 (2 × CH), 127.8 (2 × CH), 127.3 (2 × CH), 127.2 (C), 126.9 (CH), 125.3 (CH), 123.4 (CH), 120.3 (CH), 120.1 (CH), 113.6 (CH2), 113.0 (C), 111.3 (CH), 46.5 (CH2), 41.8 (CH), 21.8 (CH3). ESI-MSm/z 439 [M + Na+, 100]. Calcd for C25H24N2O2S [416.54]: C 72.09, H 5.81, N 6.73; C 72.37, H 5.88, N 6.72.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded 3g (108 mg, 69%) as a yellow wax. 1H-NMR (200 MHz, CDCl3): δ 7.55 (d, J = 8.3 Hz, 2H), 7.37–7.10 (m, 14H), 6.94 (m, 2H), 6.64 (d, J = 16.5 Hz, 1H), 4.58 (dd, J = 10.5, 6.3 Hz, 1H), 4.30 (dd, J = 8.8, 3.1 Hz, 1H), 3.84–3.59 (m, 5H), 2.39 (s, 3H), 2.34 (s, 3H). 13C-NMR (50 MHz, CDCl3): δ 143.4 (C), 142.2 (C), 138.6 (C), 138.1 (C), 137.7 (C), 136.7 (C), 135.7 (CH), 134.1 (C), 129.8 (2 × CH), 129.7 (2 × CH), 128.9 (2 × CH), 128.0 (2 × CH), 127.3 (2 × CH), 126.9 (CH), 126.8 (2 × CH), 126.6 (C), 122.4 (CH), 120.1 (CH), 119.8 (CH), 115.7 (CH), 110.7 (C), 109.8 (CH), 47.0 (CH2), 43.2 (CH), 31.1 (CH3), 21.6 (CH3), 21.5 (CH3). ESI-MS m/z 519 [M − H+, 100]. Calcd for C33H32N2O2S [520.68]: C 76.12, H 6.19, N 5.38; found C 76.23, H 6.02, N 5.49.
2) yielded 3g (108 mg, 69%) as a yellow wax. 1H-NMR (200 MHz, CDCl3): δ 7.55 (d, J = 8.3 Hz, 2H), 7.37–7.10 (m, 14H), 6.94 (m, 2H), 6.64 (d, J = 16.5 Hz, 1H), 4.58 (dd, J = 10.5, 6.3 Hz, 1H), 4.30 (dd, J = 8.8, 3.1 Hz, 1H), 3.84–3.59 (m, 5H), 2.39 (s, 3H), 2.34 (s, 3H). 13C-NMR (50 MHz, CDCl3): δ 143.4 (C), 142.2 (C), 138.6 (C), 138.1 (C), 137.7 (C), 136.7 (C), 135.7 (CH), 134.1 (C), 129.8 (2 × CH), 129.7 (2 × CH), 128.9 (2 × CH), 128.0 (2 × CH), 127.3 (2 × CH), 126.9 (CH), 126.8 (2 × CH), 126.6 (C), 122.4 (CH), 120.1 (CH), 119.8 (CH), 115.7 (CH), 110.7 (C), 109.8 (CH), 47.0 (CH2), 43.2 (CH), 31.1 (CH3), 21.6 (CH3), 21.5 (CH3). ESI-MS m/z 519 [M − H+, 100]. Calcd for C33H32N2O2S [520.68]: C 76.12, H 6.19, N 5.38; found C 76.23, H 6.02, N 5.49.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded 3h (128 mg, 87%) as a yellowish solid (m.p.: 190–192 °C). 1H-NMR (200 MHz, d6-Acetone): δ 10.59 (s, 1H), 7.70 (d, J = 8.3 Hz, 2H), 7.57–7.41 (m, 8H), 7.39–7.09 (m, 7H), 6.99 (t, J = 7.0 Hz, 1H), 6.49 (t, J = 5.5 Hz, 1H), 4.74 (t, J = 8.0 Hz, 1H), 4.08–3.80 (m, 2H), 2.34 (s, 3H). 13C-NMR (50 MHz, d6-Acetone): δ 143.0 (C), 142.6 (C), 138.4 (C), 136.9 (C), 131.3 (2 × CH), 129.7 (2 × CH), 128.9 (2 × CH), 128.5 (2 × CH), 128.2 (2 × CH), 127.1 (2 × CH), 126.8 (C), 126.6 (CH), 123.5 (CH), 123.0 (C), 121.4 (C), 119.9 (CH), 119.5 (CH), 116.8 (C), 111.5 (CH), 95.0 (C), 82.3 (C), 46.8 (CH2), 43.5 (CH), 20.7 (CH3). 1 CHar is missing, probably overlapping. ESI-MSm/z 489 [M − H+, 100]. Calcd for C31H26N2O2S [490.62]: C 75.89, H 5.34, N 5.71; found: C 76.22, H 5.28, N 5.93.
2) yielded 3h (128 mg, 87%) as a yellowish solid (m.p.: 190–192 °C). 1H-NMR (200 MHz, d6-Acetone): δ 10.59 (s, 1H), 7.70 (d, J = 8.3 Hz, 2H), 7.57–7.41 (m, 8H), 7.39–7.09 (m, 7H), 6.99 (t, J = 7.0 Hz, 1H), 6.49 (t, J = 5.5 Hz, 1H), 4.74 (t, J = 8.0 Hz, 1H), 4.08–3.80 (m, 2H), 2.34 (s, 3H). 13C-NMR (50 MHz, d6-Acetone): δ 143.0 (C), 142.6 (C), 138.4 (C), 136.9 (C), 131.3 (2 × CH), 129.7 (2 × CH), 128.9 (2 × CH), 128.5 (2 × CH), 128.2 (2 × CH), 127.1 (2 × CH), 126.8 (C), 126.6 (CH), 123.5 (CH), 123.0 (C), 121.4 (C), 119.9 (CH), 119.5 (CH), 116.8 (C), 111.5 (CH), 95.0 (C), 82.3 (C), 46.8 (CH2), 43.5 (CH), 20.7 (CH3). 1 CHar is missing, probably overlapping. ESI-MSm/z 489 [M − H+, 100]. Calcd for C31H26N2O2S [490.62]: C 75.89, H 5.34, N 5.71; found: C 76.22, H 5.28, N 5.93.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) yielded 3i (127 mg, 93%) as a white solid (m.p.: 126–128 °C). 1H-NMR (200 MHz, CDCl3): δ 8.06 (s, 1H), 7.60 (d, J = 8.3 Hz, 2H), 7.31–7.07 (m, 9H), 6.92 (t, J = 6.7 Hz, 2H), 4.48 (m, 2H), 3.80 (dd, J = 8.1, 6.3 Hz, 2H), 2.47–2.29 (m, 5H), 1.71–1.53 (m, 2H), 1.02 (t, J = 7.3 Hz, 3H). 13C-NMR (50 MHz, CDCl3): δ 143.4 (C), 141.4 (C), 137.2 (C), 135.9 (C), 129.8 (2 × CH), 128.8 (2 × CH), 128.0 (2 × CH), 127.3 (2 × CH), 127.0 (CH), 126.5 (C), 123.6 (CH), 120.3 (CH), 119.3 (CH), 118.4 (C), 118.0 (C), 111.1 (CH), 97.4 (C), 72.7 (C), 46.5 (CH2), 43.0 (CH), 22.2 (CH2), 21.8 (CH3), 21.7 (CH2), 13.9 (CH3). ESI-MSm/z 455 [M − H+, 100]. Calcd for C28H28N2O2S [456.60]: C 73.65, H 6.18, N 6.14; C 73.37, H 6.12, N 6.26.
1) yielded 3i (127 mg, 93%) as a white solid (m.p.: 126–128 °C). 1H-NMR (200 MHz, CDCl3): δ 8.06 (s, 1H), 7.60 (d, J = 8.3 Hz, 2H), 7.31–7.07 (m, 9H), 6.92 (t, J = 6.7 Hz, 2H), 4.48 (m, 2H), 3.80 (dd, J = 8.1, 6.3 Hz, 2H), 2.47–2.29 (m, 5H), 1.71–1.53 (m, 2H), 1.02 (t, J = 7.3 Hz, 3H). 13C-NMR (50 MHz, CDCl3): δ 143.4 (C), 141.4 (C), 137.2 (C), 135.9 (C), 129.8 (2 × CH), 128.8 (2 × CH), 128.0 (2 × CH), 127.3 (2 × CH), 127.0 (CH), 126.5 (C), 123.6 (CH), 120.3 (CH), 119.3 (CH), 118.4 (C), 118.0 (C), 111.1 (CH), 97.4 (C), 72.7 (C), 46.5 (CH2), 43.0 (CH), 22.2 (CH2), 21.8 (CH3), 21.7 (CH2), 13.9 (CH3). ESI-MSm/z 455 [M − H+, 100]. Calcd for C28H28N2O2S [456.60]: C 73.65, H 6.18, N 6.14; C 73.37, H 6.12, N 6.26.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) yielded 3j (103 mg, 80%) as a white wax. 1H NMR (200 MHz, CDCl3): δ 7.99 (s, 1H), 7.60 (d, J = 8.2, 2H), 7.37–7.03 (m, 10H), 6.89 (t, J = 7.5, 1H), 5.87 (m, 1H), 5.2–5.05 (m, 2H), 4.42 (dd, J = 10.4, 6.2 Hz, 1H), 4.31 (dd, J = 9.0, 3.4 Hz, 1H), 3.82 (m, 1H), 3.59 (m, 1H), 3.42 (d, J = 6.4 Hz, 2H), 2.43 (s, 3H). 13C NMR (50 MHz, CDCl3): δ 143.6 (C), 141.5 (C), 136.9 (C), 136.0 (C), 135.2 (C), 134.7 (CH), 129.9 (2 × CH), 128.7 (2 × CH), 127.9 (2 × CH), 127.3 (2 × CH), 127.2 (C), 126.8 (CH), 121.7 (CH), 119.9 (CH), 119.3 (CH), 117.9 (C), 111.2 (CH), 109.7 (CH), 46.6 (CH2), 42.2 (CH), 31.0 (CH2), 21.7 (CH3). ESI-MSm/z 429 [M − H+, 100]. Calcd for C26H26N2O2S [430.57]: C 72.53, H 6.09, N 6.51; found: C 72.87, H 5.99, N 6.61.
3) yielded 3j (103 mg, 80%) as a white wax. 1H NMR (200 MHz, CDCl3): δ 7.99 (s, 1H), 7.60 (d, J = 8.2, 2H), 7.37–7.03 (m, 10H), 6.89 (t, J = 7.5, 1H), 5.87 (m, 1H), 5.2–5.05 (m, 2H), 4.42 (dd, J = 10.4, 6.2 Hz, 1H), 4.31 (dd, J = 9.0, 3.4 Hz, 1H), 3.82 (m, 1H), 3.59 (m, 1H), 3.42 (d, J = 6.4 Hz, 2H), 2.43 (s, 3H). 13C NMR (50 MHz, CDCl3): δ 143.6 (C), 141.5 (C), 136.9 (C), 136.0 (C), 135.2 (C), 134.7 (CH), 129.9 (2 × CH), 128.7 (2 × CH), 127.9 (2 × CH), 127.3 (2 × CH), 127.2 (C), 126.8 (CH), 121.7 (CH), 119.9 (CH), 119.3 (CH), 117.9 (C), 111.2 (CH), 109.7 (CH), 46.6 (CH2), 42.2 (CH), 31.0 (CH2), 21.7 (CH3). ESI-MSm/z 429 [M − H+, 100]. Calcd for C26H26N2O2S [430.57]: C 72.53, H 6.09, N 6.51; found: C 72.87, H 5.99, N 6.61.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded 3k (100 mg, 83%) as a white solid (m.p.: 185–188 °C). 1H-NMR (200 MHz, DMSO-d6): δ 10.86 (s, 1H), 7.62 (dd, J = 8.1, 1.5 Hz, 2H), 7.39–7.05 (m, 10H), 7.00 (t, J = 8.2 Hz, 1H), 6.85 (t, J = 7.9 Hz, 1H), 4.27 (t, J = 7.7 Hz, 1H), 3.35–3.19 (m, 2H), 2.32 (s, 3H). 13C-NMR (50 MHz, DMSO-d6): δ 143.6 (C), 143.1 (C), 138.3 (C), 136.9 (C), 130.2 (2 × CH), 128.9 (2 × CH), 128.7 (2 × CH), 127.2 (2 × CH), 127.1 (C), 126.8 (CH), 122.8 (CH), 121.7 (CH), 119.0 (CH), 116.1 (C), 112.1 (CH), 48.2 (CH2), 43.3 (CH), 21.6 (CH3). ESI-MSm/z 391 [M − H+, 100]. Calcd for C23H22N2O2S [390.50]: C 70.74, H 5.68, N 7.17; found C 70.99, H 5.75, N 7.01.
2) yielded 3k (100 mg, 83%) as a white solid (m.p.: 185–188 °C). 1H-NMR (200 MHz, DMSO-d6): δ 10.86 (s, 1H), 7.62 (dd, J = 8.1, 1.5 Hz, 2H), 7.39–7.05 (m, 10H), 7.00 (t, J = 8.2 Hz, 1H), 6.85 (t, J = 7.9 Hz, 1H), 4.27 (t, J = 7.7 Hz, 1H), 3.35–3.19 (m, 2H), 2.32 (s, 3H). 13C-NMR (50 MHz, DMSO-d6): δ 143.6 (C), 143.1 (C), 138.3 (C), 136.9 (C), 130.2 (2 × CH), 128.9 (2 × CH), 128.7 (2 × CH), 127.2 (2 × CH), 127.1 (C), 126.8 (CH), 122.8 (CH), 121.7 (CH), 119.0 (CH), 116.1 (C), 112.1 (CH), 48.2 (CH2), 43.3 (CH), 21.6 (CH3). ESI-MSm/z 391 [M − H+, 100]. Calcd for C23H22N2O2S [390.50]: C 70.74, H 5.68, N 7.17; found C 70.99, H 5.75, N 7.01.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded 3l (156 mg, 97%) as a white solid (m.p.: 201–203 °C). 1H-NMR (200 MHz, CDCl3): δ 8.64 (s, 1H), 7.68 (s, 1H), 7.46–7.34 (m, 7H), 7.32–6.99 (m, 9H), 4.49 (dd, J = 10.7, 6.0 Hz, 1H), 4.13 (m, 1H), 3.69 (m, 2H), 2.40 (s, 3H). 13C-NMR (50 MHz, CDCl3): δ 143.6 (C), 141.2 (C), 140.6 (C), 136.4 (C), 135.3 (C), 131.6 (C), 129.8 (2 × CH), 129.5 (C), 129.2 (2 × CH), 129.1 (CH), 129.0 (2 × CH), 128.9 (2 × CH), 127.8 (2 × CH), 127.1 (2 × CH), 125.2 (q, J = 271 Hz, C), 124.6 (q, J = 32 Hz, C), 120.4 (CH), 116.9 (q, J = 3.5 Hz, CH), 110.5 (C), 109.1 (q, J = 4.3 Hz, CH), 46.8 (CH2), 42.2 (CH), 21.6 (CH3). ESI-MSm/z 533 [M − H+, 100]. Calcd for C30H25F3N2O2S [534.60]: C 67.40, H 4.71, N 5.24; found: C 67.76; H 4.80, N 5.31.
2) yielded 3l (156 mg, 97%) as a white solid (m.p.: 201–203 °C). 1H-NMR (200 MHz, CDCl3): δ 8.64 (s, 1H), 7.68 (s, 1H), 7.46–7.34 (m, 7H), 7.32–6.99 (m, 9H), 4.49 (dd, J = 10.7, 6.0 Hz, 1H), 4.13 (m, 1H), 3.69 (m, 2H), 2.40 (s, 3H). 13C-NMR (50 MHz, CDCl3): δ 143.6 (C), 141.2 (C), 140.6 (C), 136.4 (C), 135.3 (C), 131.6 (C), 129.8 (2 × CH), 129.5 (C), 129.2 (2 × CH), 129.1 (CH), 129.0 (2 × CH), 128.9 (2 × CH), 127.8 (2 × CH), 127.1 (2 × CH), 125.2 (q, J = 271 Hz, C), 124.6 (q, J = 32 Hz, C), 120.4 (CH), 116.9 (q, J = 3.5 Hz, CH), 110.5 (C), 109.1 (q, J = 4.3 Hz, CH), 46.8 (CH2), 42.2 (CH), 21.6 (CH3). ESI-MSm/z 533 [M − H+, 100]. Calcd for C30H25F3N2O2S [534.60]: C 67.40, H 4.71, N 5.24; found: C 67.76; H 4.80, N 5.31.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded 3m (41 mg, 30%) as a white solid (m.p.: 54–56 °C). 1H NMR (C6D6, 200 MHz): δ 7.45 (d, J = 8.4 Hz, 1H), 7.36 (d, J = 8.1 Hz, 2H), 7.19–7.12 (m, 3H), 6.98–6.89 (m, 4H), 6.58 (d, J = 8.1 Hz, 2H), 6.26–6.22 (m, 2H), 5.41 (t, J = 7.0 Hz, 1H), 4.73 (bs, 1H), 3.94–3.84 (m, 1H), 3.47–3.36 (m, 1H), 2.77 (q, J = 7.0 Hz, 4H), 1.83 (s, 3H), 0.86 (t, J = 7.0 Hz, 6H). 13C NMR (C6D6, 50 MHz): δ 145.0 (C), 142.3 (C), 140.9 (C), 137.3 (C), 135.1 (C), 129.3 (2 × CH), 129.0 (2 × CH), 127.1 (2 × CH), 126.8 (C), 126.7 (2 × CH), 124.6 (CH), 121.6 (C), 120.4 (CH), 116.1 (CH), 102.3 (CH), 49.5 (CH2), 45.8 (2 × CH2), 40.7 (CH), 20.9 (CH3), 12.8 (2 × CH3). 1 CHar is missing, probably overlapping. ESI-MSm/z 484 [M + Na+, 100], 462 [M + H+, 60]. Calcd for C27H31N3O2S: C 70.25, H 6.77, N 9.10; found C 70.15, H 6.53, N 9.07.
2) yielded 3m (41 mg, 30%) as a white solid (m.p.: 54–56 °C). 1H NMR (C6D6, 200 MHz): δ 7.45 (d, J = 8.4 Hz, 1H), 7.36 (d, J = 8.1 Hz, 2H), 7.19–7.12 (m, 3H), 6.98–6.89 (m, 4H), 6.58 (d, J = 8.1 Hz, 2H), 6.26–6.22 (m, 2H), 5.41 (t, J = 7.0 Hz, 1H), 4.73 (bs, 1H), 3.94–3.84 (m, 1H), 3.47–3.36 (m, 1H), 2.77 (q, J = 7.0 Hz, 4H), 1.83 (s, 3H), 0.86 (t, J = 7.0 Hz, 6H). 13C NMR (C6D6, 50 MHz): δ 145.0 (C), 142.3 (C), 140.9 (C), 137.3 (C), 135.1 (C), 129.3 (2 × CH), 129.0 (2 × CH), 127.1 (2 × CH), 126.8 (C), 126.7 (2 × CH), 124.6 (CH), 121.6 (C), 120.4 (CH), 116.1 (CH), 102.3 (CH), 49.5 (CH2), 45.8 (2 × CH2), 40.7 (CH), 20.9 (CH3), 12.8 (2 × CH3). 1 CHar is missing, probably overlapping. ESI-MSm/z 484 [M + Na+, 100], 462 [M + H+, 60]. Calcd for C27H31N3O2S: C 70.25, H 6.77, N 9.10; found C 70.15, H 6.53, N 9.07.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded 3n (75 mg, 54%) as a white solid (m.p.: 175–177 °C). 1H-NMR (200 MHz, CDCl3): δ 9.19 (s, 1H), 7.58 (d, J = 8.3 Hz, 2H), 7.38 (d, J = 8.2 Hz, 1H), 7.32–7.10 (m, 9H), 6.91 (t, J = 7.0 Hz, 1H), 5.39 (dd, J = 10.0, 6.1 Hz, 1H), 4.88 (m, 1H), 4.28 (q, J = 7.1 Hz, 2H), 4.00–3.70 (m, 2H), 2.39 (s, 3H), 1.37 (t, J = 7.1 Hz, 3H). 13C-NMR (50 MHz, CDCl3): δ 162.3 (C), 143.4 (C), 141.1 (C), 136.9 (C), 136.4 (C), 129.7 (2 × CH), 128.7 (2 × CH), 127.9 (2 × CH), 127.2 (2 × CH), 126.9 (CH), 126.5 (C), 125.7 (CH), 124.8 (C), 122.0 (CH), 121.9 (C), 120.8 (CH), 112.5 (CH), 61.6 (CH2), 46.6 (CH2), 41.6 (CH), 21.7 (CH3), 14.5 (CH3). ESI-MSm/z 461 [M − H+, 100]. Calcd for C26H26N2O4S [462.56]: C 67.51, H 5.67, N 6.06; found: 67.88, H. 5.52, N. 6.19.
2) yielded 3n (75 mg, 54%) as a white solid (m.p.: 175–177 °C). 1H-NMR (200 MHz, CDCl3): δ 9.19 (s, 1H), 7.58 (d, J = 8.3 Hz, 2H), 7.38 (d, J = 8.2 Hz, 1H), 7.32–7.10 (m, 9H), 6.91 (t, J = 7.0 Hz, 1H), 5.39 (dd, J = 10.0, 6.1 Hz, 1H), 4.88 (m, 1H), 4.28 (q, J = 7.1 Hz, 2H), 4.00–3.70 (m, 2H), 2.39 (s, 3H), 1.37 (t, J = 7.1 Hz, 3H). 13C-NMR (50 MHz, CDCl3): δ 162.3 (C), 143.4 (C), 141.1 (C), 136.9 (C), 136.4 (C), 129.7 (2 × CH), 128.7 (2 × CH), 127.9 (2 × CH), 127.2 (2 × CH), 126.9 (CH), 126.5 (C), 125.7 (CH), 124.8 (C), 122.0 (CH), 121.9 (C), 120.8 (CH), 112.5 (CH), 61.6 (CH2), 46.6 (CH2), 41.6 (CH), 21.7 (CH3), 14.5 (CH3). ESI-MSm/z 461 [M − H+, 100]. Calcd for C26H26N2O4S [462.56]: C 67.51, H 5.67, N 6.06; found: 67.88, H. 5.52, N. 6.19.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded 3o (75 mg, 54%) as a white solid (m.p.: 125–127 °C). 1H-NMR (200 MHz, CDCl3): δ 9.27 (s, 1H), 7.79 (d, J = 8.3 Hz, 1H), 7.57 (d, J = 8.3 Hz, 2H), 7.35–7.12 (m, 7H), 6.99 (td, J = 9.0, 2.4 Hz, 1H), 6.78 (dd, J = 9.9, 2.3 Hz, 1H), 5.33 (dd, J = 5.9, 10.4 Hz, 1H), 4.90 (m, 1H), 4.29 (q, J = 7.1 Hz, 2H), 3.89 (dt, J = 12.7, 6.4 Hz, 1H), 3.69 (td, J = 11.8, 4.6 Hz, 1H), 2.40 (s, 3H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (50 MHz, CDCl3): δ 162.0 (C), 157.8 (d, JC–F = 237 Hz, C), 143.5 (C), 140.7 (C), 136.7 (C), 133.0 (C), 129.8 (2 × CH), 128.8 (2 × CH), 127.8 (2 × CH), 127.1 (2 × CH), 127.0 (CH), 126.4 (C), 121.7 (d, JC–F = 5.5 Hz, C), 114.9 (d, JC–F = 27 Hz, CH), 113.5 (d, JC–F = 9.4 Hz, CH), 106.3 (d, JC–F = 24 Hz, CH), 61.7 (CH2), 46.2 (CH2), 41.3 (CH), 21.7 (CH3), 14.5 (CH3). ESI-MSm/z 479 [M − H+, 100]. Calcd for C26H25FN2O4S [480.55]: C 64.98, H 5.24, N 5.83; found: C 64.72, H 5.38, N 5.91.
2) yielded 3o (75 mg, 54%) as a white solid (m.p.: 125–127 °C). 1H-NMR (200 MHz, CDCl3): δ 9.27 (s, 1H), 7.79 (d, J = 8.3 Hz, 1H), 7.57 (d, J = 8.3 Hz, 2H), 7.35–7.12 (m, 7H), 6.99 (td, J = 9.0, 2.4 Hz, 1H), 6.78 (dd, J = 9.9, 2.3 Hz, 1H), 5.33 (dd, J = 5.9, 10.4 Hz, 1H), 4.90 (m, 1H), 4.29 (q, J = 7.1 Hz, 2H), 3.89 (dt, J = 12.7, 6.4 Hz, 1H), 3.69 (td, J = 11.8, 4.6 Hz, 1H), 2.40 (s, 3H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (50 MHz, CDCl3): δ 162.0 (C), 157.8 (d, JC–F = 237 Hz, C), 143.5 (C), 140.7 (C), 136.7 (C), 133.0 (C), 129.8 (2 × CH), 128.8 (2 × CH), 127.8 (2 × CH), 127.1 (2 × CH), 127.0 (CH), 126.4 (C), 121.7 (d, JC–F = 5.5 Hz, C), 114.9 (d, JC–F = 27 Hz, CH), 113.5 (d, JC–F = 9.4 Hz, CH), 106.3 (d, JC–F = 24 Hz, CH), 61.7 (CH2), 46.2 (CH2), 41.3 (CH), 21.7 (CH3), 14.5 (CH3). ESI-MSm/z 479 [M − H+, 100]. Calcd for C26H25FN2O4S [480.55]: C 64.98, H 5.24, N 5.83; found: C 64.72, H 5.38, N 5.91.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded 3p (77 mg, 66%) as a white solid (m.p.: 129–130 °C). 1H-NMR (200 MHz, CDCl3): δ 8.16 (s, 1H), 7.58 (d, J = 8.3 Hz, 2H), 7.51–7.33 (m, 7H), 7.27–7.03 (m, 4H), 4.40 (t, J = 6.0 Hz, 1H), 3.26 (m, 2H), 3.07 (m, 2H), 2.39 (s, 3H). 13C-NMR (50 MHz, CDCl3): δ 143.4 (C), 137.1 (C), 136.1(C), 135.9 (C), 132.8 (C), 129.8 (2 × CH), 129.2 (2 × CH), 128.8 (C), 128.4 (2 × CH), 128.2 (CH), 127.2 (2 × CH), 122.8 (CH), 120.2 (CH), 119.0 (CH), 111.2 (CH), 108.6 (C), 43.4 (CH2), 25.2 (CH2), 21.7 (CH3). ESI-MSm/z 389 [M − H+, 100]. Calcd for C23H22N2O2S [390.50]: C 70.74, H 5.68, N 7.17; found C 70.95, H 5.56, N 7.01.
2) yielded 3p (77 mg, 66%) as a white solid (m.p.: 129–130 °C). 1H-NMR (200 MHz, CDCl3): δ 8.16 (s, 1H), 7.58 (d, J = 8.3 Hz, 2H), 7.51–7.33 (m, 7H), 7.27–7.03 (m, 4H), 4.40 (t, J = 6.0 Hz, 1H), 3.26 (m, 2H), 3.07 (m, 2H), 2.39 (s, 3H). 13C-NMR (50 MHz, CDCl3): δ 143.4 (C), 137.1 (C), 136.1(C), 135.9 (C), 132.8 (C), 129.8 (2 × CH), 129.2 (2 × CH), 128.8 (C), 128.4 (2 × CH), 128.2 (CH), 127.2 (2 × CH), 122.8 (CH), 120.2 (CH), 119.0 (CH), 111.2 (CH), 108.6 (C), 43.4 (CH2), 25.2 (CH2), 21.7 (CH3). ESI-MSm/z 389 [M − H+, 100]. Calcd for C23H22N2O2S [390.50]: C 70.74, H 5.68, N 7.17; found C 70.95, H 5.56, N 7.01.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded 3q (96 mg, 71%) as a white solid (m.p.: 54–55 °C). 1H-NMR (200 MHz, CDCl3): δ 8.12 (s, 1H), 7.57 (d, J = 8.4 Hz, 2H), 7.44–7.31 (m, 4H), 7.22–7.01 (m, 4H), 6.93 (d, J = 8.9 Hz, 2H), 4.59 (hept, J = 6.0 Hz, 1H), 4.41 (t, J = 6.1 Hz, 1H), 3.26 (m, 2H), 3.04 (m, 2H), 2.39 (s, 3H), 1.38 (d, J = 6.1 Hz, 6H). 13C-NMR (50 MHz, CDCl3): δ 158.1 (C), 143.3 (C), 137.1 (C), 136.1 (C), 135.9 (C), 129.8 (2 × CH), 129.6 (2 × CH), 128.9 (C), 127.2 (2 × CH), 124.8 (C), 122.4 (CH), 120.1 (CH), 118.7 (CH), 116.4 (2 × CH), 111.1 (CH), 107.7 (C), 70.3 (CH), 43.4 (CH2), 25.2 (CH2), 22.3 (CH3), 21.7 (CH3). ESI-MSm/z 447 [M − H+, 100]. Calcd for C26H28N2O3S [448.58]: C 69.62, H 6.29, N 6.25; found C 69.86, H 6.32, N 6.18.
2) yielded 3q (96 mg, 71%) as a white solid (m.p.: 54–55 °C). 1H-NMR (200 MHz, CDCl3): δ 8.12 (s, 1H), 7.57 (d, J = 8.4 Hz, 2H), 7.44–7.31 (m, 4H), 7.22–7.01 (m, 4H), 6.93 (d, J = 8.9 Hz, 2H), 4.59 (hept, J = 6.0 Hz, 1H), 4.41 (t, J = 6.1 Hz, 1H), 3.26 (m, 2H), 3.04 (m, 2H), 2.39 (s, 3H), 1.38 (d, J = 6.1 Hz, 6H). 13C-NMR (50 MHz, CDCl3): δ 158.1 (C), 143.3 (C), 137.1 (C), 136.1 (C), 135.9 (C), 129.8 (2 × CH), 129.6 (2 × CH), 128.9 (C), 127.2 (2 × CH), 124.8 (C), 122.4 (CH), 120.1 (CH), 118.7 (CH), 116.4 (2 × CH), 111.1 (CH), 107.7 (C), 70.3 (CH), 43.4 (CH2), 25.2 (CH2), 22.3 (CH3), 21.7 (CH3). ESI-MSm/z 447 [M − H+, 100]. Calcd for C26H28N2O3S [448.58]: C 69.62, H 6.29, N 6.25; found C 69.86, H 6.32, N 6.18.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) yielded 3r (80 mg, 62%) as a yellowish solid (m.p.: 52.5–54 °C). 1H-NMR (200 MHz, CDCl3): δ 8.59 (s, 1H), 8.06 (m, 1H), 7.86 (d, J = 7.5 Hz, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.62–7.30 (m, 5H), 7.27–7.01 (m, 4H), 4.80 (t, J = 6.1 Hz, 1H), 3.27 (m, 2H), 3.08 (m, 2H), 2.58 (s, 3H), 2.37 (s, 3H). 13C-NMR (50 MHz, CDCl3): δ 198.4 (C), 143.5 (C), 137.8 (C), 137.1 (C), 136.3 (C), 134.7 (C), 133.3 (C), 132.8 (CH), 129.8 (2 × CH), 129.5 (CH), 128.7 (C), 127.9 (CH), 127.8 (CH), 127.2 (2 × CH), 123.1 (CH), 120.3 (CH), 119.1 (CH), 111.5 (CH), 109.3 (C), 43.5 (CH2), 26.9 (CH3), 25.5 (CH2), 21.7 (CH3). ESI-MSm/z 431 [M − H+, 100]. Calcd for C25H24N2O3S [432.53]: C 69.42, H 5.59, N 6.48; found C 69.83, H 5.47, N 6.22.
3) yielded 3r (80 mg, 62%) as a yellowish solid (m.p.: 52.5–54 °C). 1H-NMR (200 MHz, CDCl3): δ 8.59 (s, 1H), 8.06 (m, 1H), 7.86 (d, J = 7.5 Hz, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.62–7.30 (m, 5H), 7.27–7.01 (m, 4H), 4.80 (t, J = 6.1 Hz, 1H), 3.27 (m, 2H), 3.08 (m, 2H), 2.58 (s, 3H), 2.37 (s, 3H). 13C-NMR (50 MHz, CDCl3): δ 198.4 (C), 143.5 (C), 137.8 (C), 137.1 (C), 136.3 (C), 134.7 (C), 133.3 (C), 132.8 (CH), 129.8 (2 × CH), 129.5 (CH), 128.7 (C), 127.9 (CH), 127.8 (CH), 127.2 (2 × CH), 123.1 (CH), 120.3 (CH), 119.1 (CH), 111.5 (CH), 109.3 (C), 43.5 (CH2), 26.9 (CH3), 25.5 (CH2), 21.7 (CH3). ESI-MSm/z 431 [M − H+, 100]. Calcd for C25H24N2O3S [432.53]: C 69.42, H 5.59, N 6.48; found C 69.83, H 5.47, N 6.22.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded 3s (70 mg, 71%) as a white wax. 1H-NMR (200 MHz, CDCl3): δ 7.82 (s, 1H), 7.62 (d, J = 8.3 Hz, 2H), 7.33–7.16 (m, 4H), 7.14–6.96 (m, 2H), 4.31 (t, J = 6.2 Hz, 1H), 3.22 (m, 2H), 2.90 (m, 2H), 2.40 (s, 3H), 2.35 (s, 3H). 13C-NMR (50 MHz, CDCl3): δ 143.5 (C), 137.1 (C), 135.6 (C), 132.7 (C), 129.8 (2 × CH), 128.4 (C), 127.2 (2 × CH), 121.4 (CH), 119.6 (CH), 117.8 (CH), 110.7(CH), 107.3 (C), 43.4 (CH2), 24.9 (CH2), 21.7 (CH3), 11.8 (CH3). ESI-MSm/z 327 [M − H+, 65]. Calcd for C18H20N2O2S [328.43]: C 65.83, H 6.14, N 8.53; found C 66.11, H 6.00, N 8.77.
2) yielded 3s (70 mg, 71%) as a white wax. 1H-NMR (200 MHz, CDCl3): δ 7.82 (s, 1H), 7.62 (d, J = 8.3 Hz, 2H), 7.33–7.16 (m, 4H), 7.14–6.96 (m, 2H), 4.31 (t, J = 6.2 Hz, 1H), 3.22 (m, 2H), 2.90 (m, 2H), 2.40 (s, 3H), 2.35 (s, 3H). 13C-NMR (50 MHz, CDCl3): δ 143.5 (C), 137.1 (C), 135.6 (C), 132.7 (C), 129.8 (2 × CH), 128.4 (C), 127.2 (2 × CH), 121.4 (CH), 119.6 (CH), 117.8 (CH), 110.7(CH), 107.3 (C), 43.4 (CH2), 24.9 (CH2), 21.7 (CH3), 11.8 (CH3). ESI-MSm/z 327 [M − H+, 65]. Calcd for C18H20N2O2S [328.43]: C 65.83, H 6.14, N 8.53; found C 66.11, H 6.00, N 8.77.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) yielded 3t (65 mg, 69%) as a white wax. 1H-NMR (200 MHz, CDCl3): δ 8.03 (s, 1H), 7.64 (d, J = 8.3 Hz, 3H), 7.45–6.92 (m, 7H), 4.38 (t, J = 5.9 Hz, 1H), 3.28 (m, 2H), 2.93 (m, 2H), 2.40 (s, 3H). 13C-NMR (50 MHz, CDCl3): δ 143.5 (C), 137.1 (C), 136.7 (C), 129.8 (2 × CH), 127.2 (2 × CH), 122.9 (CH), 122.4 (CH), 119.7 (CH), 118.7 (CH), 111.8 (C), 111.6 (CH), 43.3 (CH2), 25.7 (CH2), 21.7 (CH3). ESI-MSm/z 313 [M − H+, 100]. Calcd for C17H18N2O2S [314.40]: C 64.94, H 5.77, N 8.91; found C 65.19, H 5.83, N 8.87.
1) yielded 3t (65 mg, 69%) as a white wax. 1H-NMR (200 MHz, CDCl3): δ 8.03 (s, 1H), 7.64 (d, J = 8.3 Hz, 3H), 7.45–6.92 (m, 7H), 4.38 (t, J = 5.9 Hz, 1H), 3.28 (m, 2H), 2.93 (m, 2H), 2.40 (s, 3H). 13C-NMR (50 MHz, CDCl3): δ 143.5 (C), 137.1 (C), 136.7 (C), 129.8 (2 × CH), 127.2 (2 × CH), 122.9 (CH), 122.4 (CH), 119.7 (CH), 118.7 (CH), 111.8 (C), 111.6 (CH), 43.3 (CH2), 25.7 (CH2), 21.7 (CH3). ESI-MSm/z 313 [M − H+, 100]. Calcd for C17H18N2O2S [314.40]: C 64.94, H 5.77, N 8.91; found C 65.19, H 5.83, N 8.87.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) yielded 3x (63 mg, 57%) as a white solid (m.p.: 46–48 °C). 1H NMR (300 MHz, CDCl3): δ 8.26 (s, 1H), 7.69–7.12 (m, 14H), 5.05 (s, 1H), 4.84 (t, J = 6.0 Hz, 1H), 3.53 (q, J = 6.8 Hz, 2H), 3.13 (t, J = 7.1 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 156.6 (C), 136.9 (C), 136.1 (C), 135.7 (C), 133.1 (C), 129.2 (C), 129.2 (2 × CH), 128.7 (CH), 128.3 (4 × CH), 128.2 (2 × CH), 128.1 (CH), 122.7 (CH), 120.1 (CH), 119.3 (CH), 111.2 (CH), 109.8 (C), 66.7 (CH2), 41.8 (CH2), 25.3 (CH2). ESI-MSm/z 393 [M + Na+, 75], 371 [M + H+, 100]. Calcd for C24H22N2O2 [370.44]: C 77.81, H 5.99, N 7.56; found C 77.69, H 5.87, N 7.68.
1) yielded 3x (63 mg, 57%) as a white solid (m.p.: 46–48 °C). 1H NMR (300 MHz, CDCl3): δ 8.26 (s, 1H), 7.69–7.12 (m, 14H), 5.05 (s, 1H), 4.84 (t, J = 6.0 Hz, 1H), 3.53 (q, J = 6.8 Hz, 2H), 3.13 (t, J = 7.1 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 156.6 (C), 136.9 (C), 136.1 (C), 135.7 (C), 133.1 (C), 129.2 (C), 129.2 (2 × CH), 128.7 (CH), 128.3 (4 × CH), 128.2 (2 × CH), 128.1 (CH), 122.7 (CH), 120.1 (CH), 119.3 (CH), 111.2 (CH), 109.8 (C), 66.7 (CH2), 41.8 (CH2), 25.3 (CH2). ESI-MSm/z 393 [M + Na+, 75], 371 [M + H+, 100]. Calcd for C24H22N2O2 [370.44]: C 77.81, H 5.99, N 7.56; found C 77.69, H 5.87, N 7.68.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded 3y (35 mg, 26%) as a white wax. 1H NMR (300 MHz, CDCl3): δ 8.02 (s, 1H), 7.51–7.39 (m, 5H), 7.29–7.18 (m, 2H), 7.15–7.08 (m, 3H), 6.84–6.77 (m, 3H), 4.15 (m, 1H), 3.43 (m, 1H), 2.83 (ddd, J = 12.2, 10.8, 4.1, 1H), 2.52 (m, 1H), 2.30 (s, 3H), 1.88 (m, 3H), 1.30 (m, 4H). 13C NMR (75 MHz, CDCl3): δ 142.6 (C), 136.4 (2 × C), 136.0 (C), 132.7 (C), 129.3 (2 × CH), 129.2 (2 × CH), 129.0 (2 × CH), 128.6 (CH), 126.6 (2 × CH), 122.1 (CH), 120.3 (CH), 119.7 (CH), 112.5 (C), 111.3 (CH), 56.3 (CH), 41.7 (CH), 35.0 (CH2), 32.5 (CH2), 26.2 (CH2), 25.3 (CH2), 21.8 (CH3) (one quaternary carbon is missing, probably overlapped with one negative signal, CH). ESI-MSm/z 444 [M + H+, 100]. Calcd for C27H28N2O2S [444.59]: C 72.94, H 6.35, N 6.30; found C 72.86, H 6.12, N 6.41.
2) yielded 3y (35 mg, 26%) as a white wax. 1H NMR (300 MHz, CDCl3): δ 8.02 (s, 1H), 7.51–7.39 (m, 5H), 7.29–7.18 (m, 2H), 7.15–7.08 (m, 3H), 6.84–6.77 (m, 3H), 4.15 (m, 1H), 3.43 (m, 1H), 2.83 (ddd, J = 12.2, 10.8, 4.1, 1H), 2.52 (m, 1H), 2.30 (s, 3H), 1.88 (m, 3H), 1.30 (m, 4H). 13C NMR (75 MHz, CDCl3): δ 142.6 (C), 136.4 (2 × C), 136.0 (C), 132.7 (C), 129.3 (2 × CH), 129.2 (2 × CH), 129.0 (2 × CH), 128.6 (CH), 126.6 (2 × CH), 122.1 (CH), 120.3 (CH), 119.7 (CH), 112.5 (C), 111.3 (CH), 56.3 (CH), 41.7 (CH), 35.0 (CH2), 32.5 (CH2), 26.2 (CH2), 25.3 (CH2), 21.8 (CH3) (one quaternary carbon is missing, probably overlapped with one negative signal, CH). ESI-MSm/z 444 [M + H+, 100]. Calcd for C27H28N2O2S [444.59]: C 72.94, H 6.35, N 6.30; found C 72.86, H 6.12, N 6.41.
Representative procedures for the ring opening reactions of 2-methyl-N-tosyl aziridine (2c and (S)2c) – see Table 2 for details
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded 3u/3′u (79.3 mg, 98%) in a 2
2) yielded 3u/3′u (79.3 mg, 98%) in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio as a white solid. The ratio and identity of the two regioisomers were established via 1D and 2D NMR analysis. See the copy of the original spectra enclosed in the ESI.† ESI-MS m/z 403 [M − H+, 100]. Reported data are in agreement with those reported in the literature.3i
1 ratio as a white solid. The ratio and identity of the two regioisomers were established via 1D and 2D NMR analysis. See the copy of the original spectra enclosed in the ESI.† ESI-MS m/z 403 [M − H+, 100]. Reported data are in agreement with those reported in the literature.3i
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded 3v/3′v (120 mg, 86%) in a 2
2) yielded 3v/3′v (120 mg, 86%) in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio as a pink solid. The ratio and identity of the two regioisomers were established by analogy with 3u/3′uvia1H NMR analysis. See the copy of the original spectra enclosed in the ESI.† ESI-MS m/z 461 [M − H+, 100].
1 ratio as a pink solid. The ratio and identity of the two regioisomers were established by analogy with 3u/3′uvia1H NMR analysis. See the copy of the original spectra enclosed in the ESI.† ESI-MS m/z 461 [M − H+, 100].
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded 3w/3′w (94.4 mg, 92%) in a 2
2) yielded 3w/3′w (94.4 mg, 92%) in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio as a pink thick oil. The ratio and identity of the two regioisomers were established by analogy with 3u/3′uvia1H NMR analysis. See the copy of the original spectra enclosed in the ESI.† ESI-MS m/z 341 [M − H+, 100].
1 ratio as a pink thick oil. The ratio and identity of the two regioisomers were established by analogy with 3u/3′uvia1H NMR analysis. See the copy of the original spectra enclosed in the ESI.† ESI-MS m/z 341 [M − H+, 100].
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded 3u and 3′u (43 mg, 55%) as inseparable mixture in a 12
2) yielded 3u and 3′u (43 mg, 55%) as inseparable mixture in a 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio as a white solid. Reported data refer to major isomers and are in agreement with those reported in ref. 3i. 1H-NMR (200 MHz, CDCl3): δ 8.10 (s, 1H), 7.49–7.30 (m, 9H), 7.26–7.15 (m, 3H), 6.99 (t, J = 7.3 Hz, 1H), 4.18 (d, J = 7.1 Hz, 1H), 3.32 (m, 3H), 2.40 (s, 3H), 1.39 (d, J = 5.9 Hz, 3H). 13C NMR (50 MHz, CDCl3): δ 143.2 (C), 136.7 (C), 136.5 (2 × C), 132.8 (C), 129.7 (2 × CH), 129.1 (2 × CH), 129.0 (2 × CH), 128.4 (CH), 127.1 (2 × CH), 126.7 (C), 122.5 (CH), 120.0 (CH), 119.9 (CH), 112.9 (C), 111.5 (CH), 48.1 (CH2), 31.8 (CH), 21.7 (CH3), 19.0 (CH3). ESI-MSm/z 427 [M + Na+, 100].
1 ratio as a white solid. Reported data refer to major isomers and are in agreement with those reported in ref. 3i. 1H-NMR (200 MHz, CDCl3): δ 8.10 (s, 1H), 7.49–7.30 (m, 9H), 7.26–7.15 (m, 3H), 6.99 (t, J = 7.3 Hz, 1H), 4.18 (d, J = 7.1 Hz, 1H), 3.32 (m, 3H), 2.40 (s, 3H), 1.39 (d, J = 5.9 Hz, 3H). 13C NMR (50 MHz, CDCl3): δ 143.2 (C), 136.7 (C), 136.5 (2 × C), 132.8 (C), 129.7 (2 × CH), 129.1 (2 × CH), 129.0 (2 × CH), 128.4 (CH), 127.1 (2 × CH), 126.7 (C), 122.5 (CH), 120.0 (CH), 119.9 (CH), 112.9 (C), 111.5 (CH), 48.1 (CH2), 31.8 (CH), 21.7 (CH3), 19.0 (CH3). ESI-MSm/z 427 [M + Na+, 100].
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielding 3u/3′u (51 mg, 70%) as an inseparable mixture in a 7
2) yielding 3u/3′u (51 mg, 70%) as an inseparable mixture in a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio as a white solid. The ratio and identity of the two regioisomers were established via1H NMR analysis and are in agreement with those reported in ref. 12. See the enclosed copy of the original spectra. The same reaction was performed using (S)-2-methyl-1-tosylaziridine ((S)2c). The purified reaction mixture was analyzed via chiral-HPLC, see the ESI† (HPLC section) for details.
1 ratio as a white solid. The ratio and identity of the two regioisomers were established via1H NMR analysis and are in agreement with those reported in ref. 12. See the enclosed copy of the original spectra. The same reaction was performed using (S)-2-methyl-1-tosylaziridine ((S)2c). The purified reaction mixture was analyzed via chiral-HPLC, see the ESI† (HPLC section) for details.
Preparation and characterization data for compounds (±)4a–d and (±)4′a–c
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) yielded progressively (±)4a and (±)4′a (170 mg, overall yield 68%, ratio 60
1) yielded progressively (±)4a and (±)4′a (170 mg, overall yield 68%, ratio 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) as white solids (m.p.: (±)4a 115–117.2 °C; (±)4′a 173–176 °C). (±)4a:161H NMR (300 MHz, CDCl3): δ 7.86 (d, J = 8.3 Hz, 2H), 7.42 (d, J = 8.0 Hz, 2H), 7.29–7.23 (m, 3H), 7.05 (dt, J = 7.7, 1.3 Hz, 1H), 6.85 (d, J = 6.2, 2H), 6.39 (d, J = 7.8 Hz, 1H), 6.31 (t, J = 7.4 Hz, 1H), 5.60 (d, J = 7.4 Hz, 1H), 5.40 (s, 1H), 3.80 (dd, J = 12.2, 6.5 Hz, 1H), 3.42 (t, J = 12.4 Hz, 1H), 3.07 (s, 3H), 2.76 (dd, J = 12.6, 6.5 Hz, 1H), 2.50 (s, 3H), 1.31 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 151.2 (C), 144.2 (C), 137.6 (C), 136.1 (C), 130.3 (2 × CH), 129.2 (2 × CH), 129.0 (C), 128.8 (CH), 128.3 (2 × CH), 127.9 (CH), 127.7 (2 × CH), 125.8 (CH), 116.8 (CH), 105.4 (CH), 91.5 (CH), 57.1 (C), 55.4 (CH), 51.6 (CH2), 31.7 (CH3), 26.4 (CH3), 22.0 (CH3). ESI(+)-MS (m/z %): 441 (100) [M + Na]+. Calcd for C25H26N2O2S [418.56]: C 71.74, H 6.26, N 6.69; found C.71.92, H 6.16, N 6.85. (±)4′a:16b1H NMR (300 MHz, CDCl3): δ 7.90 (d, J = 8.2 Hz, 2H), 7.42 (d, J = 8.1 Hz, 2H), 7.38–7.24 (m, 3H), 7.19 (t, J = 7.6 Hz, 1H), 6.96 (dd, J = 6.6, 2.9 Hz, 2H), 6.67 (t, J = 7.4 Hz, 1H), 6.56 (dd, J = 7.1, 3.5 Hz, 2H), 5.10 (s, 1H), 3.89–3.76 (m, 2H), 3.61 (dd, J = 11.0, 8.0 Hz, 1H), 3.06 (s, 3H), 2.50 (s, 3H), 0.53 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 149.5 (C), 144.4 (C), 135.7 (C), 135.5 (C), 133.0 (C), 130.2 (2 × CH), 129.1 (2 × CH), 129.0 (CH), 128.5 (2 × CH), 128.2 (2 × CH), 127.8 (CH), 122.7 (CH), 118.2 (CH), 107.9 (CH), 94.8 (CH), 54.5 (C), 52.9 (CH2), 52.4 (CH), 33.2 (CH3), 22.0 (CH3), 16.9 (CH3). ESI-MSm/z 419 [M + H+, 100]. Calcd for C25H26N2O2S [418.56]: C 71.74, H 6.26, N 6.69; found: C 71.88, H 6.02, N 6.98.
40) as white solids (m.p.: (±)4a 115–117.2 °C; (±)4′a 173–176 °C). (±)4a:161H NMR (300 MHz, CDCl3): δ 7.86 (d, J = 8.3 Hz, 2H), 7.42 (d, J = 8.0 Hz, 2H), 7.29–7.23 (m, 3H), 7.05 (dt, J = 7.7, 1.3 Hz, 1H), 6.85 (d, J = 6.2, 2H), 6.39 (d, J = 7.8 Hz, 1H), 6.31 (t, J = 7.4 Hz, 1H), 5.60 (d, J = 7.4 Hz, 1H), 5.40 (s, 1H), 3.80 (dd, J = 12.2, 6.5 Hz, 1H), 3.42 (t, J = 12.4 Hz, 1H), 3.07 (s, 3H), 2.76 (dd, J = 12.6, 6.5 Hz, 1H), 2.50 (s, 3H), 1.31 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 151.2 (C), 144.2 (C), 137.6 (C), 136.1 (C), 130.3 (2 × CH), 129.2 (2 × CH), 129.0 (C), 128.8 (CH), 128.3 (2 × CH), 127.9 (CH), 127.7 (2 × CH), 125.8 (CH), 116.8 (CH), 105.4 (CH), 91.5 (CH), 57.1 (C), 55.4 (CH), 51.6 (CH2), 31.7 (CH3), 26.4 (CH3), 22.0 (CH3). ESI(+)-MS (m/z %): 441 (100) [M + Na]+. Calcd for C25H26N2O2S [418.56]: C 71.74, H 6.26, N 6.69; found C.71.92, H 6.16, N 6.85. (±)4′a:16b1H NMR (300 MHz, CDCl3): δ 7.90 (d, J = 8.2 Hz, 2H), 7.42 (d, J = 8.1 Hz, 2H), 7.38–7.24 (m, 3H), 7.19 (t, J = 7.6 Hz, 1H), 6.96 (dd, J = 6.6, 2.9 Hz, 2H), 6.67 (t, J = 7.4 Hz, 1H), 6.56 (dd, J = 7.1, 3.5 Hz, 2H), 5.10 (s, 1H), 3.89–3.76 (m, 2H), 3.61 (dd, J = 11.0, 8.0 Hz, 1H), 3.06 (s, 3H), 2.50 (s, 3H), 0.53 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 149.5 (C), 144.4 (C), 135.7 (C), 135.5 (C), 133.0 (C), 130.2 (2 × CH), 129.1 (2 × CH), 129.0 (CH), 128.5 (2 × CH), 128.2 (2 × CH), 127.8 (CH), 122.7 (CH), 118.2 (CH), 107.9 (CH), 94.8 (CH), 54.5 (C), 52.9 (CH2), 52.4 (CH), 33.2 (CH3), 22.0 (CH3), 16.9 (CH3). ESI-MSm/z 419 [M + H+, 100]. Calcd for C25H26N2O2S [418.56]: C 71.74, H 6.26, N 6.69; found: C 71.88, H 6.02, N 6.98.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) yielded (±)4b and (±)4′b as an inseparable mixture (187 mg, overall yield 63%, ratio 60
1) yielded (±)4b and (±)4′b as an inseparable mixture (187 mg, overall yield 63%, ratio 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, white solid). (±)4b
40, white solid). (±)4b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13,14/(±)4′b: 1H-NMR (300 MHz, CDCl3): by comparison with NMR data in ref 16b, signals marked in blue and red can be attributed to (±)4b and (±)4′b, respectively. Signals in black are superimposed hydrogens. δ 7.76 (m, 4H), 7.46–7.20 (m, 20H),
13,14/(±)4′b: 1H-NMR (300 MHz, CDCl3): by comparison with NMR data in ref 16b, signals marked in blue and red can be attributed to (±)4b and (±)4′b, respectively. Signals in black are superimposed hydrogens. δ 7.76 (m, 4H), 7.46–7.20 (m, 20H),  , 7.03–6.94
, 7.03–6.94  13C-NMR (75 MHz, CDCl3): δ 150.3, 148.7, 144.2, 144.1, 139.2, 138.9, 137.3, 136.00, 135.7, 135.2, 132.9, 130.1, 130.0, 129.1 129.0, 128.8, 128.7, 128.7, 128.6, 128.4, 128.2, 128.0, 127.7, 127.6, 127.6, 127.4, 127.2, 127.0, 126.0, 122.5, 117.9, 116.7, 108.0, 105.6, 92.5, 90.1, 57.1, 55.5, 54.8, 52.8, 52.7, 51.4, 49.9, 48.3, 29.9, 26.66, 21.9, 17.0. ESI-MSm/z 495 [M + H+, 100].
13C-NMR (75 MHz, CDCl3): δ 150.3, 148.7, 144.2, 144.1, 139.2, 138.9, 137.3, 136.00, 135.7, 135.2, 132.9, 130.1, 130.0, 129.1 129.0, 128.8, 128.7, 128.7, 128.6, 128.4, 128.2, 128.0, 127.7, 127.6, 127.6, 127.4, 127.2, 127.0, 126.0, 122.5, 117.9, 116.7, 108.0, 105.6, 92.5, 90.1, 57.1, 55.5, 54.8, 52.8, 52.7, 51.4, 49.9, 48.3, 29.9, 26.66, 21.9, 17.0. ESI-MSm/z 495 [M + H+, 100].
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–9
2–9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–8
1–8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) yielded progressively (±)4′c and (±)4c (69 mg, overall yield 55%, ratio (±)4c/(±)4′c 70
2) yielded progressively (±)4′c and (±)4c (69 mg, overall yield 55%, ratio (±)4c/(±)4′c 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) as white solids (m.p.: (±)4c 177–179 °C; (±)4′c 145–147 °C). (±)4c: 1H-NMR (300 MHz, CDCl3): δ 7.72 (d, J = 8.1 Hz, 2H), 7.41–6.95 (m, 8H), 6.56 (t, J = 7.8 Hz, 1H), 6.46 (t, J = 7.3 Hz, 1H), 5.92 (d, J = 7.4 Hz, 1H), 5.42 (bs, 1H), 3.58–3.32 (m, 3H), 2.39 (s, 3H), 1.84 (s, 3H), 1.22 (s, 3H). 13C-NMR (75 MHz, CDCl3): δ 148.2 (C), 143.3 (C), 139.1 (C), 137.6 (C), 135.6 (C), 129.7 (2 × CH), 129.6 (2 × CH), 128.3 (2 × CH), 127.9 (CH), 127.7 (CH), 127.3 (2 × CH), 126.1 (CH), 118.4 (CH), 109.2 (CH), 91.9 (C), 59.5 (C), 51.4 (CH), 50.6 (CH2), 23.5 (CH3), 22.7 (CH3), 21.7 (CH3). ESI-MSm/z 419 [M + H+, 100]. Calcd for C25H26N2O2S [418.56]: C 71.74, H 6.26, N 6.69; found: C 71.95, H 6.02, N 6.81. (±)4′c: 1H-NMR (300 MHz, CDCl3): δ 7.80 (d, J = 8.3 Hz, 2H), 7.32–7.23 (m, 5H), 7.15–7.08 (m, 2H), 7.06 (dd, J = 7.6, 1.1 Hz, 1H), 6.99 (d, J = 6.8 Hz, 1H), 6.76 (dt, J = 7.4, 0.8 Hz, 1H), 6.58 (d, J = 7.8 Hz, 1H), 5.26 (bs, 1H), 3.70 (dd, J = 7.5, 2.1 Hz, 1H), 3.47 (m, 2H), 2.43 (s, 3H), 1.72 (s, 3H), 0.93 (s, 3H). 13C-NMR (75 MHz, CDCl3): δ 146.9 (C), 143.6 (C), 139.3 (C), 137.3 (C), 135.0 (C), 129.9 (2 × CH), 129.1 (2 × CH), 128.7 (CH), 128.5 (2 × CH), 127.9 (2 × CH), 127.5 (CH), 122.8 (CH), 119.4 (CH), 109.5 (CH), 92.3 (C), 59.8 (C), 52.6 (CH2), 51.6 (CH), 24.1 (CH3), 21.9 (CH3), 19.7 (CH3). ESI-MSm/z 419 [M + H+, 100]. Calcd for C25H26N2O2S [418.56]: C 71.74, H 6.26, N 6.69; found: C 71.90, H 6.11, N 6.58.
30) as white solids (m.p.: (±)4c 177–179 °C; (±)4′c 145–147 °C). (±)4c: 1H-NMR (300 MHz, CDCl3): δ 7.72 (d, J = 8.1 Hz, 2H), 7.41–6.95 (m, 8H), 6.56 (t, J = 7.8 Hz, 1H), 6.46 (t, J = 7.3 Hz, 1H), 5.92 (d, J = 7.4 Hz, 1H), 5.42 (bs, 1H), 3.58–3.32 (m, 3H), 2.39 (s, 3H), 1.84 (s, 3H), 1.22 (s, 3H). 13C-NMR (75 MHz, CDCl3): δ 148.2 (C), 143.3 (C), 139.1 (C), 137.6 (C), 135.6 (C), 129.7 (2 × CH), 129.6 (2 × CH), 128.3 (2 × CH), 127.9 (CH), 127.7 (CH), 127.3 (2 × CH), 126.1 (CH), 118.4 (CH), 109.2 (CH), 91.9 (C), 59.5 (C), 51.4 (CH), 50.6 (CH2), 23.5 (CH3), 22.7 (CH3), 21.7 (CH3). ESI-MSm/z 419 [M + H+, 100]. Calcd for C25H26N2O2S [418.56]: C 71.74, H 6.26, N 6.69; found: C 71.95, H 6.02, N 6.81. (±)4′c: 1H-NMR (300 MHz, CDCl3): δ 7.80 (d, J = 8.3 Hz, 2H), 7.32–7.23 (m, 5H), 7.15–7.08 (m, 2H), 7.06 (dd, J = 7.6, 1.1 Hz, 1H), 6.99 (d, J = 6.8 Hz, 1H), 6.76 (dt, J = 7.4, 0.8 Hz, 1H), 6.58 (d, J = 7.8 Hz, 1H), 5.26 (bs, 1H), 3.70 (dd, J = 7.5, 2.1 Hz, 1H), 3.47 (m, 2H), 2.43 (s, 3H), 1.72 (s, 3H), 0.93 (s, 3H). 13C-NMR (75 MHz, CDCl3): δ 146.9 (C), 143.6 (C), 139.3 (C), 137.3 (C), 135.0 (C), 129.9 (2 × CH), 129.1 (2 × CH), 128.7 (CH), 128.5 (2 × CH), 127.9 (2 × CH), 127.5 (CH), 122.8 (CH), 119.4 (CH), 109.5 (CH), 92.3 (C), 59.8 (C), 52.6 (CH2), 51.6 (CH), 24.1 (CH3), 21.9 (CH3), 19.7 (CH3). ESI-MSm/z 419 [M + H+, 100]. Calcd for C25H26N2O2S [418.56]: C 71.74, H 6.26, N 6.69; found: C 71.90, H 6.11, N 6.58.
Notes and references
-
(a) G. Callebaut, T. Meiresonne, N. De Kimpe and S. Mangelinckx, Chem. Rev., 2014, 114, 7954 CrossRef CAS PubMed
; (b) P.-A. Wang, Beilstein J. Org. Chem., 2013, 9, 1677 CrossRef PubMed
; (c) R. Chawla, A. K. Singh and L. D. S. Yadav, RSC Adv., 2013, 3, 11385 RSC
; (d) S. Stanković, M. D'hooghe, S. Catak, H. Eum, M. Waroquier, V. Van Speybroeck, N. De Kimpe and H.-J. Ha, Chem. Soc. Rev., 2012, 41, 643 RSC
; (e) S. H. Krake and S. C. Bergmeier, Tetrahedron, 2010, 66, 7337 CrossRef CAS
; (f) P. Lu, Tetrahedron, 2010, 66, 2549 CrossRef CAS
; (g) G. S. Singh, M. D'hooghe and N. De Kimpe, Chem. Rev., 2007, 107, 2080 CrossRef CAS PubMed
.
-
(a) M. Ishikura, T. Abe, T. Choshi and S. Hibino, Nat. Prod. Rep., 2015, 32, 1389 RSC
; (b) S. Lancianesi, A. Palmieri and M. Petrini, Chem. Rev., 2014, 114, 7108 CrossRef CAS PubMed
; (c) A. N. Da Silva and S. J. Tepper, CNS Drugs, 2012, 26, 823 CrossRef CAS PubMed
; (d) D. G. Barceloux, in Medical Toxicology of Drug Abuse, John Wiley & Sons, Inc., Hoboken, 2012, pp. 193–199 Search PubMed
; (e) D. Zhang, H. Song and Y. Qin, Acc. Chem. Res., 2011, 44, 447 CrossRef CAS PubMed
; (f) A. J. Kochanowska-Karamyan and M. T. Hamann, Chem. Rev., 2010, 110, 4489 CrossRef CAS PubMed
; (g) G. Blay, J. R. Pedro and C. Vila, in Catalytic Asymmetric Friedel-Crafts Alkylations, ed. M. Bandini and A. Umani-Ronchi, Wiley-VCH, Weinheim, 2009, pp. 223–270 Search PubMed
.
- Selected recent examples:
(a) M. K. Ghorai, D. P. Tiwari and N. Jain, J. Org. Chem., 2013, 78, 7121 CrossRef CAS PubMed
; (b) I. Tirotta, N. L. Fifer, J. Eakins and C. A. Hutton, Tetrahedron Lett., 2013, 54, 618 CrossRef CAS
; (c) J. Cockrell, C. Wilhelmsen, H. Rubin, A. Martin and J. B. Morgan, Angew. Chem., Int. Ed., 2012, 51, 9842 CrossRef CAS PubMed
; (d) Y. Li, E. Hayman, M. Plesescu and S. R. Prakash, Tetrahedron Lett., 2008, 49, 1480 CrossRef CAS
; (e) H. M. Kaiser, W. F. Lo, A. M. Riahi, A. Spannenberg, M. Beller and M. K. Tse, Org. Lett., 2006, 8, 5761 CrossRef CAS PubMed
; (f) T. Hudlicky, U. Rinner, K. J. Finn and I. Ghiviriga, J. Org. Chem., 2005, 70, 3490 CrossRef CAS PubMed
; (g) U. Rinner, T. Hudlicky, H. Gordon and G. R. Pettit, Angew. Chem., Int. Ed., 2004, 43, 5342 CrossRef CAS PubMed
; (h) J. S. Yadav, B. V. S. Reddy and G. Parimala, J. Chem. Res., Synop., 2003, 78 CrossRef CAS
; (i) R. N. Farr, R. J. Alabaster, J. Y. L. Chung, B. Craig, J. S. Edwards, H. W. Gibson, G.-J. Ho, G. R. Humphrey, S. A. Johnson and E. J. J. Grabowski, Tetrahedron: Asymmetry, 2003, 14, 3503 CrossRef CAS
; (j) T. Nishikawa, S. Kajii, K. Wada, M. Ishikawa and M. Isobe, Synthesis, 2002, 1658 CAS
; (k) J. S. Yadav, B. V. S. Reddy, S. Abraham and G. Sabitha, Tetrahedron Lett., 2002, 43, 1565 CrossRef CAS
.
- For selected recent reviews and books on indole synthesis see:
(a) G. Bartoli, R. Dalpozzo and M. Nardi, Chem. Soc. Rev., 2014, 43, 4728 RSC
; (b) G. Abbiati, F. Marinelli, E. Rossi and A. Arcadi, Isr. J. Chem., 2013, 53, 856 CrossRef CAS
; (c) M. Platon, R. Amardeil, L. Djakovitch and J.-C. Hierso, Chem. Soc. Rev., 2012, 41, 3929 RSC
; (d) S. Cacchi and G. Fabrizi, Chem. Rev., 2011, 111, PR215 CrossRef PubMed
; (e) R. Vicente, Org. Biomol. Chem., 2011, 9, 6469 RSC
; (f) S. Cacchi, G. Fabrizi and A. Goggiamani, Org. Biomol. Chem., 2011, 9, 641 RSC
; (g) S. A. Patil, R. Patil and D. D. Miller, Curr. Med. Chem., 2011, 18, 615 CrossRef CAS PubMed
; (h) J. Barluenga, F. Rodríguez and F. J. Fañanás, Chem. – Asian J., 2009, 4, 1036 CrossRef CAS PubMed
; (i) N. T. Patil and Y. Yamamoto, Chem. Rev., 2008, 108, 3395 CrossRef CAS PubMed
; (j) K. Krüger, A. Tillack and M. Beller, Adv. Synth. Catal., 2008, 350, 2153 CrossRef
; (k) L. Ackermann, Synlett, 2007, 507 CrossRef CAS
; (l) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875 CrossRef CAS PubMed
; (m) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS PubMed
; (n) G. Zeni and R. C. Larock, Chem. Rev., 2004, 104, 2285 CrossRef CAS PubMed
; (o) Modern Heterocyclic Chemistry, ed. J. Barluenga and C. Valdés, Wiley-VCH Verlag GmbH & Co. KGaA, 2011, pp. 377–531 Search PubMed
.
- For selected recent reviews and books on indole functionalization see:
(a) R. Dalpozzo, Chem. Soc. Rev., 2015, 44, 742 RSC
; (b) M. Shiri, Chem. Rev., 2012, 112, 3508 CrossRef CAS PubMed
; (c) M. Bandini, Org. Biomol. Chem., 2013, 11, 5206 RSC
; (d) G. Broggini, E. M. Beccalli, A. Fasana and S. Gazzola, Beilstein J. Org. Chem., 2012, 8, 1730 CrossRef CAS PubMed
; (e) T. Lindel, N. Marsch and S. K. Adla, Top. Curr. Chem., 2012, 309, 67 CrossRef CAS PubMed
; (f) C. C. J. Loh and D. Enders, Angew. Chem., Int. Ed., 2012, 51, 46 CrossRef CAS PubMed
; (g) N. Batail, M. Genelot, V. Dufaud, L. Joucla and L. Djakovitch, Catal. Today, 2011, 173, 1 CrossRef
; (h) M. Bandini, Chem. Soc. Rev., 2011, 40, 1358 RSC
; (i) E. M. Beck and M. J. Gaunt, Top. Curr. Chem., 2010, 292, 85 CrossRef CAS PubMed
; (j) A. Palmieri, M. Petrini and R. R. Shaikh, Org. Biomol. Chem., 2010, 8, 1259 RSC
; (k) G. Bartoli, G. Bencivenni and R. Dalpozzo, Chem. Soc. Rev., 2010, 39, 4449 RSC
; (l) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9608 CrossRef CAS PubMed
; (m) L. Joucla and L. Djakovitch, Adv. Synth. Catal., 2009, 351, 673 CrossRef CAS
; (n) Catalytic Asymmetric Friedel–Crafts Alkylations, ed. M. Bandini and A. Umani-Ronchi, Wiley-VCH, Weinheim, 2009 Search PubMed
; (o) B. Thomas, T. B. Poulsen and K. A. Jørgensen, Chem. Rev., 2008, 108, 2903 CrossRef PubMed
.
- For selected recent reviews on indole dearomatization see:
(a) W. Zi, Z. Zuo and D. Ma, Acc. Chem. Res., 2015, 48, 702 CrossRef CAS PubMed
; (b) Q. Ding, X. Zhou and R. Fan, Org. Biol. Chem., 2014, 12, 4807 RSC
. For selected recent examples on indole dearomatization see: (c) W. Zi, H. Wu and F. D. Toste, J. Am. Chem. Soc., 2015, 137, 3225 CrossRef CAS PubMed
; (d) C. Liu, Q. Yin, L.-X. Dai and S.-L. You, Chem. Commun., 2015, 51, 5971 RSC
; (e) M. Jia, M. Monari, Q.-Q. Yang and M. Bandini, Chem. Commun., 2015, 51, 2320 RSC
; (f) Y.-C. Zhang, J.-J. Zhao, F. Jiang, S.-B. Sun and F. Shi, Angew. Chem., Int. Ed., 2014, 53, 13912 CrossRef CAS PubMed
; (g) M. Mari, S. Lucarini, F. Bartoccini, G. Piersanti and G. Spadoni, Beilstein J. Org. Chem., 2014, 10, 1991 CrossRef PubMed
; (h) M. Jia, G. Cera, D. Perrotta, M. Monari and M. Bandini, Chem. – Eur. J., 2014, 20, 9875 CrossRef CAS PubMed
; (i) H. Li, R. P. Hughes and J. Wu, J. Am. Chem. Soc., 2014, 136, 6288 CrossRef CAS PubMed
; (j) H. Yin, T. Wang and N. Jiao, Org. Lett., 2014, 16, 2302 CrossRef CAS PubMed
; (k) L. Han, C. Liu, W. Zhang, X.-X. Shi and S.-L. You, Chem. Commun., 2014, 50, 1231 RSC
.
- For selected recent reviews on polycyclic indole synthesis see:
(a) L.-Q. Lu, J.-R. Chen and W.-J. Xiao, Acc. Chem. Res., 2012, 45, 1278 CrossRef CAS PubMed
. For selected recent papers on polycyclic indole synthesis see: (b) C. N. Rao, D. Lentz and H.-U. Reissig, Angew. Chem., Int. Ed., 2015, 54, 2750 CrossRef CAS PubMed
; (c) J. Gao, Y. Shao, J. Zhu, J. Zhu, H. Mao, X. Wang and X. Lv, J. Org. Chem., 2014, 79, 9000 CrossRef CAS PubMed
; (d) R. K. Arigela, R. Kumar, S. Samala, S. Gupta and B. Kundu, Eur. J. Org. Chem., 2014, 6057 CrossRef CAS
; (e) C. R. Reddy, U. Dilipkumarand and M. D. Reddy, Org. Lett., 2014, 16, 3792 CrossRef PubMed
; (f) S. Das, R. L. Harde, D. E. Shelke, N. Khairatkar-Joshi, M. Bajpai, R. S. Sapalya, H. V. Surve, G. S. Gudi, R. Pattem, D. B. Behera, B. J. Satyawan and A. Thomas, Bioorg. Med. Chem. Lett., 2014, 24, 2073 CrossRef CAS PubMed
; (g) S. He, R. P. Hsung, W. R. Presser, Z.-X. Ma and B. J. Haugen, Org. Lett., 2014, 16, 2180 CrossRef CAS PubMed
; (h) T. Wang, S. Shi, D. Pflaesterer, E. Rettenmeier, M. Rudolph, F. Rominger and A. S. K. Hashmi, Chem. – Eur. J., 2014, 20, 292 CrossRef CAS PubMed
.
-
(a) V. Pirovano, M. Dell'Acqua, D. Facoetti, S. Rizzato, G. Abbiati and E. Rossi, Eur. J. Org. Chem., 2013, 6267 CrossRef CAS
; (b) V. Pirovano, L. Decataldo, E. Rossi and R. Vicente, Chem. Commun., 2013, 49, 3594 RSC
; (c) E. Rossi, V. Pirovano, M. Negrato, G. Abbiati and M. Dell'Acqua, Beilstein J. Org. Chem., 2015, 11, 1997 CrossRef CAS PubMed
; (d) V. Pirovano, E. Arpini, M. Dell'Acqua, R. Vicente, G. Abbiati and E. Rossi, Adv. Synth. Catal., 2016, 358, 403 CrossRef CAS
.
- V. Pirovano, D. Facoetti, M. Dell'Acqua, E. Della Fontana, G. Abbiati and E. Rossi, Org. Lett., 2013, 15, 3812 CrossRef CAS PubMed
.
- I.-P. Lorenz, C. Krinninger, R. Wilberger, R. Bobka, H. Piotrowski, M. Warchhold and H. Nöth, J. Organomet. Chem., 2005, 690, 1986 CrossRef CAS
.
- X. Sun, W. Sun, R. Fan and J. Wu, Adv. Synth. Catal., 2007, 349, 2151 CrossRef CAS
.
-
(a) J. J. Hirner, K. E. Roth, Y. Shi and S. A. Blum, Organometallics, 2012, 31, 6843 CrossRef CAS PubMed
; (b) Y.-Q. Zhang, Z.-H. Chen, Y.-Q. Tu, C.-A. Fan, F.-M. Zhang, A.-X. Wang and D.-Y. Yuan, Chem. Commun., 2009, 2706 RSC
; (c) S. Miaskiewicz, J.-M. Weibel, P. Pale and A. Blanc, Org. Lett., 2016, 18, 844 CrossRef CAS PubMed
; (d) M. Hoffmann, J.-M. Weibel, P. de Fremont, P. Pale and A. Blanc, Org. Lett., 2014, 16, 908 CrossRef CAS PubMed
; (e) N. Kern, M. Hoffmann, A. Blanc, J.-M. Weibel and P. Pale, Org. Lett., 2013, 15, 836 CrossRef CAS PubMed
; (f) N. Kern, A. Blanc, S. Miaskiewicz, M. Robinette, J.-M. Weibel and P. Pale, J. Org. Chem., 2012, 77, 4323 CrossRef CAS PubMed
; (g) N. Kern, A. Blanc, J.-M. Weibel and P. Pale, Chem. Commun., 2011, 47, 6665 RSC
.
- See the ESI† for details.
- M. Nakagawa and M. Kawahara, Org. Lett., 2000, 2, 953 CrossRef CAS PubMed
.
-
(a) D. Yang, L. Wang, F. Han, D. Li, D. Zhao, Y. Cao, Y. Ma, W. Kong, Q. Sun and R. Wang, Chem. – Eur. J., 2014, 20, 16478 CrossRef CAS PubMed
; (b) L. Wang, D. Yang, F. Han, D. Li, D. Zhao and R. Wang, Org. Lett., 2015, 17, 176 CrossRef CAS PubMed
.
-
(a) Y.-M. Huang, C.-W. Zheng, L. Pan, Q.-W. Jin and G. Zhao, J. Org. Chem., 2015, 80, 10710 CrossRef CAS PubMed
; (b) Z. Chai, Y.-M. Zhu, P.-J. Yang, S. Wang, S. Wang, Z. Liu and G. Yang, J. Am. Chem. Soc., 2015, 137, 10088 CrossRef CAS PubMed
.
- The stereochemistry of the indoline products was tentatively assigned by analogy with the results reported by Zhao et al. (ref. 16a). Thus, they synthesized almost pure (S,S,S)3a, (R,R,R)3a and (S,S,S)3b whose structures were established by single-crystal X-ray diffraction.
-
(a) J. J. Hirner, K. E. Roth, Y. Shi and S. A. Blum, Organometallics, 2012, 31, 6843 CrossRef CAS PubMed
; (b) N. Kern, A. Blanc, S. Miaskiewicz, M. Robinette, J.-M. Weibel and P. Pale, J. Org. Chem., 2012, 77, 4323 CrossRef CAS PubMed
.
- M. K. Ghorai, A. Kumar and D. Prakash Tiwari, J. Org. Chem., 2010, 75, 137 CrossRef CAS PubMed
.
- E. Rossi, G. Abbiati, V. Canevari, E. Magri and G. Celentano, Synthesis, 2006, 299 CrossRef CAS
.
-
(a) C. Shao, G. Shi, Y. Zhang, S. Pan and X. Guan, Org. Lett., 2015, 17, 2652 CrossRef CAS PubMed
; (b) J. S. Yadav, B. V. S. Reddy, P. Muralikrishna Reddy and Ch. Srinivas, Tetrahedron Lett., 2002, 43, 5185 CrossRef CAS
; (c) I. Usui, S. Schmidt, M. Keller and B. Breit, Org. Lett., 2008, 10, 1207 CrossRef CAS PubMed
.
- M. Nagamochi, Y.-Q. Fang and M. Lautens, Org. Lett., 2007, 9, 2955 CrossRef CAS PubMed
.
- T. Pei, Y.-C. Chen, P. G. Dormer and I. W. Davies, Angew. Chem., Int. Ed., 2008, 47, 4231 CrossRef CAS PubMed
.
- J. Waser, B. Gaspar, H. Nambu and E. M. Carreira, J. Am. Chem. Soc., 2006, 128, 11693 CrossRef CAS PubMed
.
-
(a) R. A. Craig, N. R. O'Connor, A. F. G. Goldberg and B. M. Stoltz, Chem. – Eur. J., 2014, 20, 4806 CrossRef CAS PubMed
; (b) Y. Pei, K. Brade, E. Brule, L. Hagberg, F. Lake and C. Moberg, Eur. J. Org. Chem., 2005, 2835 CrossRef CAS
; (c) T. A. Moss, D. M. Barber, A. F. Kyle and D. J. Dixon, Chem. – Eur. J., 2013, 19, 3071 CrossRef CAS PubMed
; (d) M. Žinić, S. Alihodžić and V. Škarić, J. Chem. Soc., Perkin Trans. 1, 1993, 21 RSC
; (e) H. Rubin, J. Cockrell and J. B. Morgan, J. Org. Chem., 2013, 78, 8865 CrossRef CAS PubMed
; (f) N. Hsueh, G. J. Clarkson and M. Shipman, Org. Lett., 2015, 17, 3632 CrossRef CAS PubMed
.
-
(a) N. Mézailles, L. Ricard and F. Gagosz, Org. Lett., 2005, 7, 4133 CrossRef PubMed
; (b) L. Ricard and F. Gagosz, Organometallics, 2007, 26, 4704 CrossRef CAS
.
- M. Dell'Acqua, L. Ronda, R. Piano, S. Pellegrino, F. Clerici, E. Rossi, A. Mozzarelli, M. L. Gelmi and G. Abbiati, J. Org. Chem., 2015, 80, 10939 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ob00672h |
| This journal is © The Royal Society of Chemistry 2016 |