The effect of photoisomerization on the enzymatic hydrolysis of polymeric micelles bearing photo-responsive azobenzene groups at their cores†
Abstract
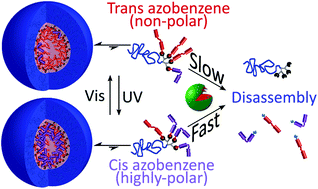
The design of stable polymeric micelles that can respond to specific stimuli is crucial for the development of smart micellar nanocarriers that can release their active cargo selectively at the target site, thus diminishing the therapeutic limitations due to non-selective damage to healthy tissues. Here we report the design and synthesis of photo- and enzyme-responsive amphiphilic PEG–dendron hybrids bearing one, two or four enzymatically cleavable azobenzene end-groups. These dual-responsive hybrids can respond to light through the reversible isomerization of the azobenzene end-groups from the non-polar trans isomer to the highly polar cis isomer and vice versa, upon UV and visible irradiation, respectively. The high structural precision of these hybrids, which emerges from the dendritic architecture, enabled a detailed study of the photoisomerization of the azobenzene end-groups with high molecular resolution. Remarkably, although the transition from trans-to-cis led to a significant increase in the polarity of the micellar cores, the micelles remained stable. Our kinetic studies show that although the trans isomer is a better substrate for the activating enzyme, the UV induced formation of the cis azobenzene end-groups led to significant acceleration of the enzymatic hydrolysis of the end-groups. These results provide strong indication that the enzyme cannot reach the core of the micelles and instead the end-groups have to leave the hydrophobic core in order to be exposed on the micelle's surface or even leave the micelle in order to allow their cleavage by the activating enzymes.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...