Mechanochemical solid-state synthesis of 2-aminothiazoles, quinoxalines and benzoylbenzofurans from ketones by one-pot sequential acid- and base-mediated reactions†
Abstract
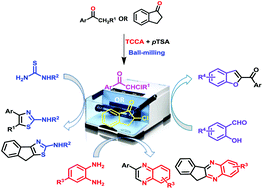
α-Chloroketones – obtained by the atom-economical chlorination of ketones with trichloroisocyanuric acid (TCCA) in the presence of p-TSA under ball-milling conditions – were set up for a sequential base-mediated condensation reaction with thiourea/thiosemicarbazides, o-phenylenediamine and salicylaldehyde to afford 2-aminothiazoles, 2-hydrazinylthiazoles, quinoxalines and benzoylbenzofurans, respectively, in respectable yields. The viability of one-pot sequential acid- and base-mediated reactions in the solid state under ball-milling conditions is thus demonstrated.

 Please wait while we load your content...
Please wait while we load your content...