Open Access Article
Open Access ArticleOne-pot relay catalysis: divergent synthesis of furo[3,4-b]indoles and cyclopenta[b]indoles from 3-(2-aminophenyl)-1,4-enynols†
Manisha
,
Seema
Dhiman
,
Jopaul
Mathew
and
S. S. V.
Ramasastry
*
Organic Synthesis and Catalysis Lab, Department of Chemical Sciences, Indian Institute of Science Education and Research (IISER) Mohali, Knowledge City, Sector 81, S. A. S. Nagar, Manuali PO, Punjab 140306, India. E-mail: ramsastry@iisermohali.ac.in; ramsastrys@gmail.com; Web: http://14.139.227.202/faculty/sastry/
First published on 25th February 2016
Abstract
Described herein is an efficient divergent strategy for the synthesis of furo[3,4-b]indoles via a sequential Ag(I)/Bi(III)/Pd(II) catalysis and cyclopenta[b]indoles via a one-pot Ag(I)/Brønsted acid relay catalysis from 3-(2-aminophenyl)-4-pentenyn-3-ols, accessible in three simple steps from 2-aminobenzaldehydes.
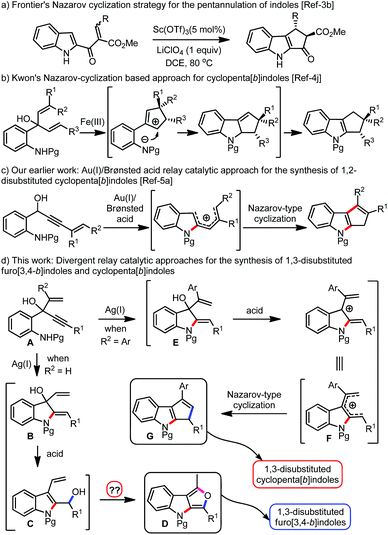
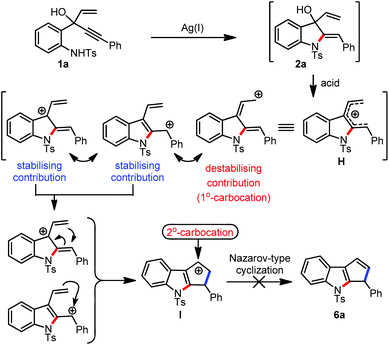
Indoles and indolines are considered privileged scaffolds from the standpoint of drug discovery.1 Among several indole derivatives, cyclopentannulated indoles have attracted great attention from synthetic chemists owing to their occurrence in several natural products and pharmaceutically interesting compounds.2 Consequently, several synthetic strategies were developed for cyclopenta[b]annulated indoles.3 Among them, the Nazarov reaction-based approaches are popular (see for example, Scheme 1a and b). An organized generation of a 4π-electron system is the key to accomplishing the Nazarov cyclization effectively.4 In this context, we have recently reported a one-pot gold(I)/Brønsted acid relay catalytic methodology toward the synthesis of a variety of 1,2-disubstituted cyclopenta[b]indoles, Scheme 1c.5a
Relay catalysis has emerged as a powerful synthetic tool to assemble complex molecular architectures in a short and efficient manner.6 Multi-catalyst-promoted processes can be atom-economical and thus can minimize the difficulties involved in isolation and purification of the intermediates. But the development of such processes is not always straightforward. Compatibility issues between the catalytic systems and control over the selectivity aspects complicate the advancement of such methods. Among the several existing categories, novel relay processes facilitated by two distinct metal catalysts or metal/organocatalyst binary systems have been well-explored.7 However, to the best of our knowledge, reactions promoted by three orthogonal metal relay catalytic systems are not reported so far.8 Herein, we report our efforts towards developing a new synthetic approach for furo[3,4-b]indoles via a Ag(I)/Bi(III)/Pd(II)-promoted relay catalytic system that integrates an intramolecular hydroamination, 1,3-allylic alcohol isomerization (1,3-AAI),8d and an unprecedented isofuran annulation of δ,ε-unsaturated alcohols, Scheme 1d. In addition, we also report a Ag(I)/Brønsted acid relay system that facilitates the synthesis of cyclopenta[b]indoles via an intramolecular hydroamination and Nazarov-type cyclization cascade, Scheme 1d.
It was envisioned that the designer substrates A could generate B by undergoing a Ag(I)-catalyzed 5-exo-dig cyclization. Subsequent acid promoted 1,3-allylic alcohol isomerization (1,3-AAI) and intramolecular etherification through a 5-exo-trig cyclization of C, especially under oxidative conditions, could afford the furo[3,4-b]indoles D, Scheme 1d.
On the other hand, the 5-exo-dig product E under acidic conditions could generate a 4π-electron system F, which by undergoing a Nazarov-type cyclization could provide access to cyclopenta[b]indoles G, Scheme 1d.
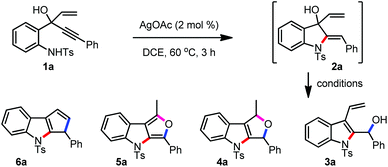
With this background and in continuation of our efforts towards the construction of cyclopentannulated arenes and heteroarenes,5a,9 we started evaluating the generality of the hypothesis presented in Scheme 1d. Accordingly, we have initiated screening of various reagents and solvent combinations with 1a as the model substrate, with the intention to obtain the pentannulated indole 6a, Table 1.10
| Entry | Conditions | Yieldb (%) |
|---|---|---|
| a Reaction conditions: see the ESI. b Isolated yields after column chromatography. c Reaction did not reach completion. d Yield based on starting material recovery. e Reaction performed with the isolated sample of 3a. | ||
| 1 | Bi(OTf)3 (10 mol%), DCE, 2 h, 0 °C–RT | 3a (85) |
| 2 | La(OTf)3 (10 mol%), DCE, 24 h, 0 °C–RT | 3a (68) |
| 3 | Yb(OTf)3 (10 mol%), DCE, 24 h, 0 °C–RT | 3a (64) |
| 4 | PTSA (10 mol%), DCE, 12 h, 0 °C | 4a (40) |
| 5c | CSA (10 mol%), DCE, 48 h, 0 °C | 4a (35) |
| 6 | TfOH (10 mol%), DCE, 4 h, 0 °C | 4a (—) |
| 7 | PTSA (10 mol%), DCM, 13 h, RT | 4a (35) |
| 8 | PTSA (10 mol%), toluene, 15 h, RT | 4a (12) |
| 9 | PTSA (10 mol%), MeNO2, 48 h, RT | 4a (—) |
| 10 | PTSA (10 mol%), DCE, 3 h, 60 °C | 4a (35) |
| 11 | PTSA (10 mol%), DCE, 72 h, −20 °C | 4a (—) |
| 12d | PTSA (10 mol%), DCE, 11 h, RT | 4a (45) |
| 13 | (i) Bi(OTf)3 (5 mol%), DCE, 3 h, RT; (ii) Pd(OAc)2 (10 mol%), DCE, 20 h, RT | 5a (45) |
| 14 | (i) Bi(OTf)3 (5 mol%), DCE, 3 h, RT; (ii) Pd(OAc)2 (10 mol%), DCE, 30 h, 0 °C | 5a (43) |
| 15e | (i) Bi(OTf) 3 (5 mol%), DCE, 3 h, RT; (ii) Pd(OAc) 2 (10 mol%), DCE, 10 h, 60 °C | 5a (50) |
| 16 | (i) Bi(OTf)3 (5 mol%), DCE, 3 h, RT; (ii) PdCl2 (10 mol%), DCE, 10 h, 60 °C | 5a (—) |
| 17 | (i) Bi(OTf)3 (5 mol%), DCE, 3 h, RT; (ii) Pd(dppf)Cl2 (10 mol%), DCE, 10 h, 60 °C | 5a (—) |
| 18 | (i) Bi(OTf)3 (5 mol%), DCE, 3 h, RT; (ii) CuI (10 mol%), DCE, 4 h, RT | 5a (—) |
| 19 | (i) Bi(OTf)3 (5 mol%), DCE, 3 h, RT; (ii) AgNO3 (10 mol%), DCE, 4 h, RT | 5a (—) |
To begin with, the Au(I)-catalyzed intramolecular hydroamination conditions reported earlier by our group were tried for 2a.5a However, no desired product was observed. Among the few other variations attempted, to our delight, AgOAc successfully delivered the 5-exo-dig product 2a in excellent chemo- and regioselectivities.11 The stage is now set for identifying suitable conditions to ensure the formation of 6a.
The reaction of 2a catalysed by Lewis acids such as the triflates of Bi, La, and Yb generated only the indolyl alcohol 3a in good yields (Table 1, entries 1–3). The subsequent evaluation of Lewis acids such as TMSOTf, BF3OEt2, and FeCl3 gave only a complex mixture. Interestingly, under the influence of p-toluenesulfonic acid (PTSA) and camphorsulfonic acid (CSA), formation of the dihydrofuro[3,4-b]indole 4a was observed, which presumably formed via the 5-exo-trig cyclization of 3a (Table 1, entries 4 and 5). Despite realizing 4a in moderate yields, we were pleased to establish a new one-pot approach for the synthesis of 1,3-disubstituted 3,4-dihydro-1H-furo[3,4-b]indoles.12 Several subsequent efforts directed to improve the yield of 4a were met with no considerable success (Table 1, entries 6–11), except for a marginal yield increment in the case when the PTSA reaction was performed with the purified sample of 3a (Table 1, entry 12).
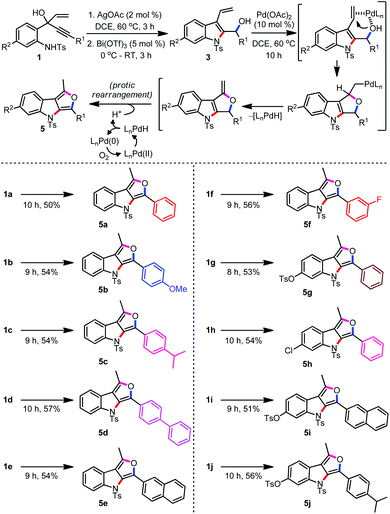
As part of the attempts to improve the efficiency of the formation of 4a from 2a, we made yet another significant observation. The reaction of 3a, obtained via Bi(OTf)3 promoted the reaction of 2a, in the presence of a catalytic amount of Pd(OAc)2 delivered the furo[3,4-b]indole 5a in 45% yield (Table 1, entry 13). Furo[3,4-b]indoles serve as an indole-2,3-quinodimethane synthetic analogue in diverse inter- and intramolecular Diels–Alder reactions. These intermediates have been applied for the synthesis of novel classes of heterocycles such as benzocarbazoles, pyridocarbazoles etc., including the antitumor alkaloid ellipticine.13
Among few other variations undertaken to increase the efficiency of the reaction, only the reaction of 3a at an elevated temperature provided 5a with a slight increase in the yield (Table 1, entry 15). Employing a few other Pd(II), Cu(II) or Ag(I) salts proved to be unsuccessful (Table 1, entries 16–19). Low yields of 4a or 5a, in general, are attributed to their inherent instability, which is well-documented in the literature.13
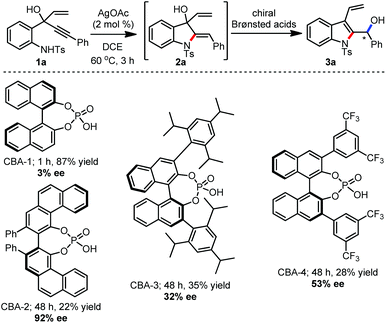
A brief screening of chiral Brønsted acids was undertaken for the one-pot enantioselective synthesis of 3a, Scheme 2.14 For this, chiral phosphoric acids CBA-1 to CBA-4 were evaluated for the transformation of 2a to 3a. With the (R)-VAPOL hydrogen phosphate CBA-2, 3a was realized in 92% ee, however, the reaction was found to be stalling after a certain extent of conversion. On the other hand, 3a was obtained in 87% yield with (R)-BINOL phosphoric acid, but almost as a racemic mixture. Several of our attempts to achieve a better result were futile. Nevertheless, a new one-pot relay Ag(I)/chiral Brønsted acid system was established for the enantioselective synthesis of 3a.
With the optimised conditions for furo[3,4-b]indoles in hand, we proceeded to evaluate the substrate scope, Table 2.15 Evidently, a wide range of structurally and electronically diverse substituents across the alkyne (R1) and the aryl ring (R2) were well-tolerated and generated the furoindoles 5a–5j in moderate to good yields. A narrow yield range (50–56%) indicates the robustness of the method which in turn highlights the least dependence of the relay catalytic system on the electron-donating/withdrawing contributions of the substituents. Interestingly, the furoindoles 5 were isolated in poor yields when the reaction was carried out in a one-pot trimetallic relay mode [Ag(I)/Bi(III)/Pd(II)], better yields were observed by performing the reaction initially under a one-pot bimetallic Ag(I)/Bi(III) system followed by subjecting the purified indolyl alcohols 3 to Pd(II) catalysis. Nevertheless, this method can serve as a potential alternative to the existing approaches describing the synthesis of the interesting and short-lived furo[3,4-b]indoles.
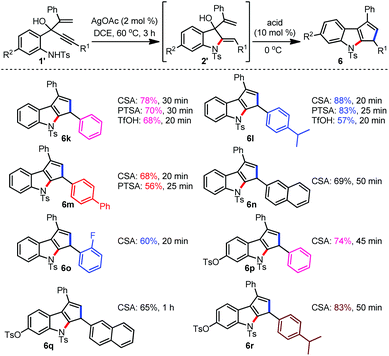
After successfully establishing a new method for the synthesis of furo[3,4-b]indoles, we turned our attention to rationalise the non-formation of 6a from 1a. A mechanistic hypothesis for this observation is proposed in Scheme 3. The indoline 2a under the influence of an acid generates the 4π-electron system H, which during the process of undergoing a Nazarov-type cyclization builds up the potentially destabilising 2°-carbocationic intermediate I. So, an additional substitution at this position creates a more stabilized 3°-carbocationic intermediate that can pave the way for the formation of the desired Nazarov cyclised product. Based on this hypothesis, we have prepared the enynol 1′k which now possesses a phenyl group at the vinylic position.15 The phenyl group at this position can also play a pivotal role in shifting the system from s-trans to s-cis in the pentadienyl cation intermediate H.
Among the few Lewis and Brønsted acids evaluated for the conversion of indoline 2′k to the Nazarov product 6k, PTSA, CSA and TfOH were found to be optimum. So, the initial reactions were performed with all the three Brønsted acids to assess their consistency. It can be realised from Table 3 that the pentannulated indoles 6k, 6l and 6m formed consistently in excellent yields under the CSA catalysis. So, CSA was chosen as the catalyst of choice for further deliberations. A subsequent evaluation of the substrate scope under the optimised conditions furnished a variety of 1,3-disubstituted cyclopenta[b]indoles 6n–6r in very good yields.16 This methodology thus can provide an efficient and robust synthetic access to several bioactive natural products and medicinally important compounds possessing a cyclopenta[b]indole scaffold.
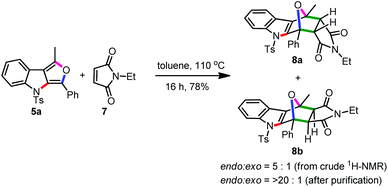
Having established new one-pot relay catalytic approaches for the furo[3,4-b]indoles and cyclopenta[b]indoles, we planned an elaboration with the former class. Earlier, Gribble and co-workers demonstrated that furo[3,4-b]indoles behave very well as dienes in the Diels–Alder reaction, and this strategy was widely employed in the construction of several complex molecular architectures.13a,b,e,f For this, the furoindole 5a and N-ethylmaleimide 7 were refluxed in toluene to produce a mixture of endo/exo Diels–Alder adducts (8a and 8b) in 78% yield, Scheme 4. The isomeric structures were assigned from the 1H and 13C-NMR data and further verified from the reported data of similar structures.13b This result indirectly confirms the structure of 5a and other analogues as well.
Interestingly, the reaction of furoindole 5c with N-ethylmaleimide 7 under toluene reflux conditions furnished, via the [4 + 2] adduct 8c, the carbazole 9c in good yield, Scheme 5.13 Carbazoles are considered privileged structures from the standpoint of medicinal chemistry. Carbazole scaffold is present in several bioactive natural products, various pharmaceuticals and functional materials.17 This method therefore can provide an access to highly functionalized carbazoles as well.
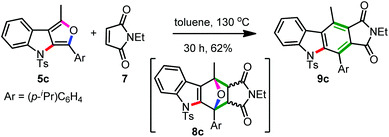 | ||
| Scheme 5 Formation of the carbazole 9cvia the Diels–Alder reaction of the furoindole 5c and maleimide 7. | ||
Conclusions
In summary, we have presented for the first time, a divergent one-pot relay catalytic approach for the synthesis of 1,3-disubstituted furo[3,4-b]indoles and cyclopenta[b]indoles starting from easily accessible 3-(2-aminophenyl)-4-pentenyn-3-ols. Interesting substitution dependence on the product distribution was realised. Based on the mechanistic considerations, this phenomenon was efficiently crafted to yield the product of choice. The methodologies described herein have demonstrated great potential and will stimulate further research in the synthesis of new heterocycles. We are currently involved in extending these strategies towards the total synthesis of complex indole-based natural products and the results will be communicated in due course.Acknowledgements
This work was supported financially by the Department of Science and Technology (DST), Govt. of India (SR/FT/CS-156/2011) and IISER Mohali. We thank the NMR and mass facilities at IISER Mohali. Manisha and J. M. thank DST for the INSPIRE fellowship and S. D. thanks IISER Mohali for a research fellowship.Notes and references
- Some selected reviews: (a) M. Somei and F. Yamada, Nat. Prod. Rep., 2005, 22, 73 RSC; (b) T. Kawasaki and K. Higuchi, Nat. Prod. Rep., 2005, 22, 761 RSC; (c) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS PubMed; (d) K. Higuchi and T. Kawasaki, Nat. Prod. Rep., 2007, 24, 843 RSC; (e) V. Sharma, P. Kumar and D. Pathak, J. Heterocycl. Chem., 2010, 47, 491 CAS; (f) A. J. Kochanowska-Karamyan and M. T. Hamann, Chem. Rev., 2010, 110, 4489 CrossRef CAS PubMed.
- For spiroindimicins, see: (a) W. Zhang, Z. Liu, S. Li, T. Yang, Q. Zhang, L. Ma, X. Tian, H. Zhang, C. Huang, S. Zhang, J. Ju, Y. Shen and C. Zhang, Org. Lett., 2012, 14, 3364 CrossRef CAS PubMed. For fischerindoles, see: (b) J. M. Richter, Y. Ishihara, T. Masuda, B. W. Whitefield, T. Llamas, A. Pohjakallio and P. S. Baran, J. Am. Chem. Soc., 2008, 130, 17938 CrossRef CAS PubMed. For yeuhchukene, see: (c) Y.-C. Kong, K.-F. Cheng, R. C. Cambie and P. G. Waterman, J. Chem. Soc., Chem. Commun., 1986, 47 Search PubMed. For polyveolines, see: (d) I. Ngantchou, B. Nyasse, C. Denier, C. Blonski, V. Hannaert and B. Schneider, Bioorg. Med. Chem. Lett., 2010, 20, 3495 CrossRef CAS PubMed. For MK-0524, see: (e) C. F. Sturino, G. O. Neill, N. Lachance, M. Boyd, C. Berthelette, M. Labelle, L. Li, B. Roy, J. Scheigetz, N. Tsou, Y. Aubin, K. P. Bateman, N. Chauret, S. H. Day, J. F. L. Vesque, C. Seto, J. H. Silva, L. A. Trimble, M. C. Carriere, D. Denis, G. Greig, S. Kargman, S. Lamontagne, M. C. Mathieu, N. Sawyer, D. Slipetz, W. M. Abraham, T. Jones, M. McAuliffe, H. Piechuta, D. A. N. Griffith, Z. Wang, R. Zamboni, R. N. Young and K. M. Metters, J. Med. Chem., 2007, 50, 794 CrossRef CAS PubMed.
- For some recent references about cyclopenta[b]indoles, see: (a) E. M. Ferreira and B. M. Stoltz, J. Am. Chem. Soc., 2003, 125, 9578 CrossRef CAS PubMed; (b) J. A. Malona, J. M. Colbourne and A. J. Frontier, Org. Lett., 2006, 8, 5661 CrossRef CAS PubMed; (c) A. K. Yadav, S. Peruncheralathan, H. Ila and H. Junjappa, J. Org. Chem., 2007, 72, 1388 CrossRef CAS PubMed; (d) C. Ferrer, C. H. M. Amijs and A. M. Echavarren, Chem. – Eur. J., 2007, 13, 1358 CrossRef CAS PubMed; (e) E. P. Balskus and C. T. Walsh, J. Am. Chem. Soc., 2009, 131, 14648 CrossRef CAS PubMed; (f) N.-W. Tseng and M. Lautens, J. Org. Chem., 2009, 74, 1809 CrossRef CAS PubMed; (g) K. Saito, H. Sogou, T. Suga, H. Kusama and N. Iwasawa, J. Am. Chem. Soc., 2011, 133, 689 CrossRef CAS PubMed; (h) B. Chen, W. Fan, G. Chai and S. Ma, Org. Lett., 2012, 14, 3616 CrossRef CAS PubMed; (i) B. Xu, Z.-L. Guo, W.-Y. Jin, Z.-P. Wang, Y.-G. Peng and Q.-X. Guo, Angew. Chem., Int. Ed., 2012, 51, 1059 CrossRef CAS PubMed; (j) S. D. Jacob, J. L. Brooks and A. J. Frontier, J. Org. Chem., 2014, 79, 10296 CrossRef CAS PubMed; (k) W. Zi, H. Wu and F. D. Toste, J. Am. Chem. Soc., 2015, 137, 3225 CrossRef CAS PubMed; (l) K. S. Feldman, I. Y. Gonzalez and C. M. Glinkerman, J. Org. Chem., 2015, 80, 11849 CrossRef CAS PubMed.
- A few selected articles on the Nazarov cyclizations, see: (a) I. N. Nazarov and I. I. Zaretskaya, Izv. Akad. Nauk. SSSR, Ser. Khim., 1941, 211 CAS; (b) H. Pellissier, Tetrahedron, 2005, 61, 6479 CrossRef CAS; (c) A. J. Frontier and C. Collison, Tetrahedron, 2005, 61, 7577 CrossRef CAS; (d) M. Tius, Eur. J. Org. Chem., 2005, 2193 CrossRef CAS; (e) L. Zhang and S. Wang, J. Am. Chem. Soc., 2006, 128, 1442 CrossRef CAS PubMed; (f) W. Nakanishi and F. G. West, Curr. Opin. Drug Discovery Dev., 2009, 12, 732 CAS; (g) W. T. Spencer III, T. Vaidya and A. J. Frontier, Eur. J. Org. Chem., 2013, 3621 CrossRef PubMed; (h) M. A. Tius, Chem. Soc. Rev., 2014, 43, 2979 RSC; (i) D. R. Wenz and J. R. de Alaniz, Eur. J. Org. Chem., 2015, 23 CrossRef CAS; (j) Z. Wang, X. Xu, Z. Gu, W. Feng, H. Qian, Z. Li, X. Sun and O. Kwon, Chem. Commun., 2016, 52, 2811 RSC.
- (a) S. Dhiman and S. S. V. Ramasastry, Org. Lett., 2015, 17, 5116 CrossRef CAS PubMed. For the applications of gold catalysis in organic synthesis, see: (b) A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896 CrossRef PubMed; (c) D. Pflästerer and A. S. K. Hashmi, Chem. Soc. Rev., 2016, 45, 1331 RSC.
- For some selected articles on relay catalysis, see: (a) D. E. Fogg and E. N. dos Santos, Coord. Chem. Rev., 2004, 248, 2365 CrossRef CAS; (b) A. Ajamian and J. L. Gleason, Angew. Chem., Int. Ed., 2004, 43, 3754 CrossRef CAS PubMed; (c) A. De Meijere, P. von Zezschwitz and S. Brase, Acc. Chem. Res., 2005, 38, 413 CrossRef CAS PubMed; (d) J.-C. Wasilke, S. J. Obrey, R. T. Baker and G. C. Bazan, Chem. Rev., 2005, 105, 1001 CrossRef CAS PubMed; (e) D. B. Ramachary and R. Sakthidevi, Org. Biomol. Chem., 2008, 6, 2488 RSC; (f) A. Grossmann and D. Enders, Angew. Chem., Int. Ed., 2012, 51, 314 CrossRef CAS PubMed; (g) N. T. Patil, V. S. Shinde and B. Gajula, Org. Biomol. Chem., 2012, 10, 211 RSC; (h) S. Fustero, E. Rodriguez, R. Lazaro, L. Herrera, S. Catalan and P. Barrio, Adv. Synth. Catal., 2013, 355, 1058 CrossRef CAS.
- (a) P. C. Too, S. H. Chua, S. H. Wong and S. Chiba, J. Org. Chem., 2011, 76, 6159 CrossRef CAS PubMed; (b) Y.-F. Wang, K. K. Toh, J.-Y. Lee and S. Chiba, Angew. Chem., Int. Ed., 2011, 50, 5927 CrossRef CAS PubMed; (c) S. Zhang, Z. Xu, J. Jia, C.-H. Tung and Z. Xu, Chem. Commun., 2014, 50, 12084 RSC; (d) X. Wang, S. Dong, Z. Yao, L. Feng, P. Daka, H. Wang and Z. Xu, Org. Lett., 2014, 16, 22 CrossRef CAS PubMed; (e) Q. Gao, P. Zhou, F. Liu, W. J. Hao, C. Yao, B. Jiang and S.-J. Tu, Chem. Commun., 2015, 51, 9519 RSC; (f) H. Peng, N. G. Akhmedov, Y.-F. Liang, N. Jiao and X. Shi, J. Am. Chem. Soc., 2015, 137, 8912 CrossRef CAS PubMed; (g) B. Wang, Y. Chen, L. Zhou, J. Wang and Z. Xu, Org. Biomol. Chem., 2016, 14, 826 RSC.
- For some one-pot triple relay catalytic systems, see: (a) G. Dong, P. Teo, Z. K. Wickens and R. H. Grubbs, Science, 2011, 333, 1609 CrossRef CAS PubMed; (b) N. T. Patil, V. S. Raut and R. B. Tella, Chem. Commun., 2013, 49, 570 RSC; (c) X.-P. Yin, X.-P. Zeng, Y.-L. Liu, F.-M. Liao, J.-S. Yu, F. Zhou and J. Zhou, Angew. Chem., Int. Ed., 2014, 53, 13740 CrossRef CAS PubMed; (d) S. Dhiman, U. K. Mishra and S. S. V. Ramasastry, Angew. Chem., Int. Ed., 2016 DOI:10.1002/anie.201600840.
- (a) B. Satpathi, S. Dhiman and S. S. V. Ramasastry, Eur. J. Org. Chem., 2014, 2022 CrossRef CAS; (b) S. Dhiman and S. S. V. Ramasastry, Chem. Commun., 2015, 51, 557 RSC; (c) B. Satpathi and S. S. V. Ramasastry, Angew. Chem., Int. Ed., 2016, 55, 1777 CrossRef CAS PubMed.
- See ESI† for the synthesis of the enynol 1a and other analogous enynols employed in this study.
- D. Susanti, F. Koh, J. A. Kusuma, P. Kothandaraman and P. W. H. Chan, J. Org. Chem., 2012, 77, 7166 CrossRef CAS PubMed.
- To the best of our knowledge, no general methods are known for the synthesis of 3,4-dihydro-1H-furo[3,4-b]indoles of the type 4a.
- (a) G. W. Gribble, M. G. Saulnier, M. P. Sibi and J. A. Obaza-Nutaitis, J. Org. Chem., 1984, 49, 4518 CrossRef CAS; (b) G. W. Gribble, D. J. Keavy, D. A. Davis, M. G. Saulnier, B. Pelcman, T. C. Barden, M. P. Sibi, E. R. Olson and J. J. BelBruno, J. Org. Chem., 1992, 57, 5878 CrossRef CAS; (c) M. T. Díaz, A. Cobas, E. Guitián and L. Castedo, Synlett, 1997, 157 Search PubMed; (d) S.-C. Lin, F.-D. Yang, J.-S. Shiue, S.-M. Yang and J.-M. Fang, J. Org. Chem., 1998, 63, 2909 CrossRef CAS; (e) G. W. Gribble, R. A. Silva and M. G. Saulnier, Synth. Commun., 1999, 29, 729 CrossRef CAS; (f) G. W. Gribble, J. Jiang and Y. Liu, J. Org. Chem., 2002, 67, 1001 CrossRef CAS PubMed; (g) J. Basset, M. Romero, T. Serra and M. D. Pujol, Tetrahedron, 2012, 68, 356 CrossRef CAS.
- H. Wu, Y.-P. He and F. Shi, Synthesis, 2015, 1990 CrossRef CAS.
- For a detailed discussion on the substitution effects in the Nazarov reactions, see: (a) A. P. Marcus, A. S. Lee, R. L. Davis, D. J. Tantillo and R. Sarpong, Angew. Chem., Int. Ed., 2008, 47, 6379 CrossRef CAS PubMed; (b) C. D. Smith, G. Rosocha, L. Mui and R. A. Batey, J. Org. Chem., 2010, 75, 4716 CAS and references cited therein. For a related reference, see: (c) J. E. Camp, D. Craig, K. Funai and A. J. P. White, Org. Biomol. Chem., 2011, 9, 7904 RSC.
- For our earlier work on the synthesis of 1,2,3-trisubstituted cyclopenta[b]indoles, see: ref. 9b. For our earlier work on the synthesis of 1,2-disubstituted cyclopenta[b]indoles, see: ref. 5a.
- Few selected references: (a) J. Bergman and B. Pelcman, Pure Appl. Chem., 1990, 62, 1967 CrossRef CAS; (b) C. J. Moody, Synlett, 1994, 681 CrossRef CAS; (c) J. Roy, A. K. Jana and D. Mal, Tetrahedron, 2012, 68, 6099 CrossRef CAS; (d) K. Higuchi and T. Kawasaki, Nat. Prod. Rep., 2007, 24, 843 RSC; (e) A. W. Schmidt, K. R. Reddy and H. J. Knolker, Chem. Rev., 2012, 112, 3193 CrossRef CAS PubMed; (f) N. Yoshikai and Y. Wei, Asian J. Org. Chem., 2013, 2, 466 CrossRef CAS; (g) K. S. Rathore, M. Harode and S. Katukojvala, Org. Biomol. Chem., 2014, 12, 8641 RSC and references cited therein.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterisation data for new compounds. See DOI: 10.1039/c6ob00319b |
| This journal is © The Royal Society of Chemistry 2016 |