Open Access Article
Open Access ArticleProbing the self-assembly and stability of oligohistidine based rod-like micelles by aggregation induced luminescence†
Hendrik
Frisch
a,
Daniel
Spitzer
a,
Mathias
Haase
b,
Thomas
Basché
b,
Jens
Voskuhl
*c and
Pol
Besenius
 *a
*a
aInstitute of Organic Chemistry, Johannes Gutenberg-Universität Mainz, Duesbergweg 10-14, D-55128 Mainz, Germany. E-mail: besenius@uni-mainz.de; Tel: +49-6131-39-22355
bInstitute of Physical Chemistry, Johannes Gutenberg-Universität Mainz, Duesbergweg 10-14, D-55128 Mainz, Germany
cInstitute of Organic Chemistry, University of Duisburg-Essen, Universitätsstrasse 7, D-45117 Essen, Germany. E-mail: jens.voskuhl@uni-due.de; Tel: +49-201-183-2404
First published on 8th March 2016
Abstract
The synthesis and self-assembly of a new C2-symmetric oligohistidine amphiphile equipped with an aggregation induced emission luminophore is reported. We observe the formation of highly stable and ordered rod-like micelles in phosphate buffered saline, with a critical aggregation concentration below 200 nM. Aggregation induced emission of the luminophore confirms the high stability of the anisotropic assemblies in serum.
Self-assembly of small molecules into macromolecular and polymeric architectures in water has been an increasingly popular synthetic strategy in organic materials chemistry since the pioneering work on supramolecular polymers 25 years ago.1,2 The use of directional non-covalent interactions in the molecular subunits can yield highly ordered one-dimensional supramolecular assemblies.3–9 These display dynamic behaviour and possess biological function that are not accessible through conventional covalent polymers.10 Particularly powerful design motifs to produce nanoscopic and ordered polymers in water are synthetic oligopeptides, using for example cyclic designs,11,12 aliphatic13–15 or aromatic amphiphiles,16,17 and dendritic oligopeptides.18,19 Promising biological function20 arises through applications as drug delivery vehicles,21–28 vaccines,29–32 imaging agents33,34 or supports for tissue engineering.15,35–38
To date, we have focused our attention on preparing dynamic supramolecular nanorods obtained via the self-assembly of C3-symmetric amphiphilic peptides,19,39–42 and luminescent spherical micelles or 2D-sheets based on linear Au(I)-metallopeptides.43 Here we report a new C2-symmetric oligohistidine amphiphile equipped with an aggregation induced emission luminophore.44–49 The development of intrinsically luminescent supramolecular assemblies is of high interest, since it avoids the need for additional labelling of monomers. In addition self-assembling luminophores that operate in luminescence “turn-on” mode further allow one to probe and track self-assembled states in more complex media,50,51 for example serum where highly abundant proteins make the use of electron microscopy and conventional luminescence “turn-off” probes very challenging.
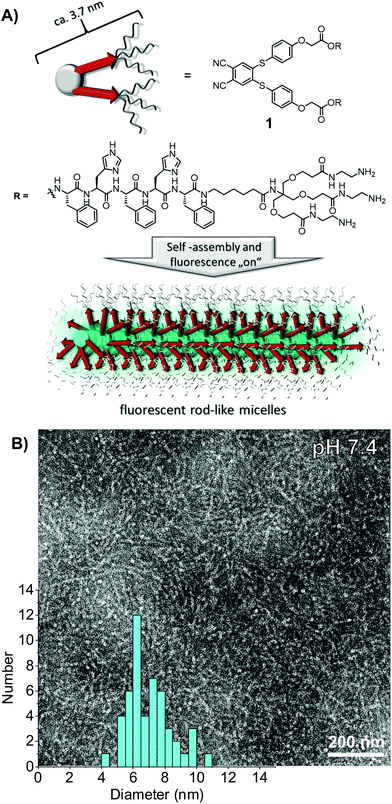
The self-assembly unit is based on a C2-symmetric 4,5-bis-(phenylthio)phthalonitrile (BPTP) as the central hydrophobic moiety extended with a β-sheet encoded alternating phenyl alanine–histidine peptide sequence (FHFHF) which bears a C-terminal hydrophilic polycationic dendron (Fig. 1A). The synthetic route is based on a convergent approach.52 We have incorporated hydrophobic FH alternating amino acid sequences in the side arm of the self-assembly unit, including an apolar hexyl-spacer separating the hydrophobic block from the water solubilising dendritic amine block (Fig. 1). The BPTP luminescent core was prepared by reacting 4,5-dichlorophthalonitrile first with 4-hydroxythiophenol, then with bromo-tert-butyl acetic acid in two high yielding steps. After deprotection of the C-terminal carboxylic acid, PyBOP mediated amidation of 4 with the protected dendronised peptide 8, and subsequent deprotection of the histidine as well as the primary amine functional groups yielded the target molecule 1. The detailed synthesis, purification and full characterisation of all intermediates can be found in the ESI.†
In order to investigate the morphology of self-assembled C2-symmetrical peptide amphiphile 1, we carried out negative stain transmission electron microscopy (TEM) experiments.40 Solutions of 1 at 100 μM concentrations in Tris buffer (10 mM) at pH 7.4 showed one-dimensional semiflexible objects with a length that could not be resolved (>500 nm) and an average thickness of 7.0 ± 1.4 nm (Fig. 1B). The diameter of the rods is twice as large as expected compared to the approximated chain extended length of a single molecule of about 3.7 nm. The semi-flexible rods are therefore best described as micellar-like arrangement of amphiphiles with the hydrophobic block pointing into the core of one-dimensional assemblies, solvated via the cationic dendrons in the shell.
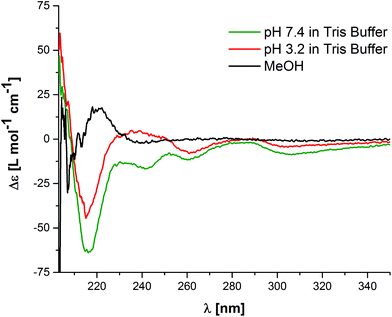
Circular dichroism (CD) spectroscopy experiments were carried out in order to gain insight into the molecular order of these nanostructures. The CD spectra of solutions of 1 analysed via TEM showed a minimum at around 216 nm, which is characteristic of supramolecular assemblies with a high content of β-sheet secondary structure (Fig. 2).53 This indicates that the high level of molecular order leading to the formation of anisotropic nanostructures arises from the formation of hydrogen-bonded β-sheet domains along the fibre axis, which is well known for the self-assembly of peptide amphiphiles.13,54,55
In order to confirm that the self-assembly is driven by the hydrophobicity of the BPTP moiety and the peptide sequence, assisted by hydrogen bonding we performed disassembly experiments using a chemical denaturant. The addition of an UV-transparent organic solvent like methanol leads to the disruption of the hydrophobic shielding of the hydrogen bonding sequences and therefore disassembly of 1.39,41,42 CD spectra show the disappearance of the β-sheet characteristic negative CD band at 216 nm and a new positive CD band at 220 nm appears, which we have previously assigned to molecularly dissolved building blocks based on alternating hydrophobic and hydrophilic sequence pairs of phenyl alanine and glutamic acid (FEFEF) or phenyl alanine and lysine (FKFKF).40,52 It is interesting to point out that by lowering the pH value, we could not observe the same changes in the CD spectra compared to the spectra recorded in methanol. We initially expected the protonation of the histidine to increase the hydrophilicity of the peptide side arms and thereby induce disassembly, similar to our previous work on the C3-symmetric FXFXF sequences. Note that a reduction in the β-sheet band at 216 nm by 31% is indicative of a decrease in the degree of order, but not full disassembly, likely due to protonation and swelling in the peptide core. These pH-titrations are indicative of highly stable assemblies. In summary, the TEM and CD experiments in buffered aqueous media demonstrate the self-assembly into ordered and stable rod-like micelles.
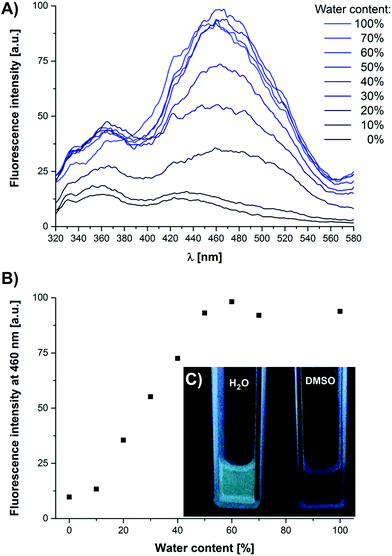
To investigate whether the luminescence properties of BPTP can be utilised to probe the self-assembly of 1 in various media, we first of all performed a series of photoluminescence spectroscopy measurements of 1 in DMSO/H2O mixtures (Fig. 3A). Similarly to MeOH,‡ DMSO is an efficient denaturant since it is known to disrupt hydrogen bonded assemblies in water.56,57 Upon increasing the water content in DMSO from 0–40 vol% we observe an increase in the emission band at λem = 460 nm (Fig. 3B). At 50 vol% H2O the emission intensity reaches a plateau value, which is indicative of a fully aggregated state. The observed aggregation induced emission (AIE) of 1 most likely arises from a combination of the restriction of intramolecular motions (RIM)49 and twisted intramolecular charge transfer (TICT).58 In the aggregated state the intramolecular rotations and vibrations of 1 are restricted, blocking radiationless relaxation channels and promoting the radiative decay pathway. Furthermore, solvatochromic effects like TICT could influence the luminescence, since in DMSO the molecularly dissolved 1 is exposed to a highly polar environment, while upon aggregation in H2O the luminophore is embedded in the apolar inner core of the micelles.
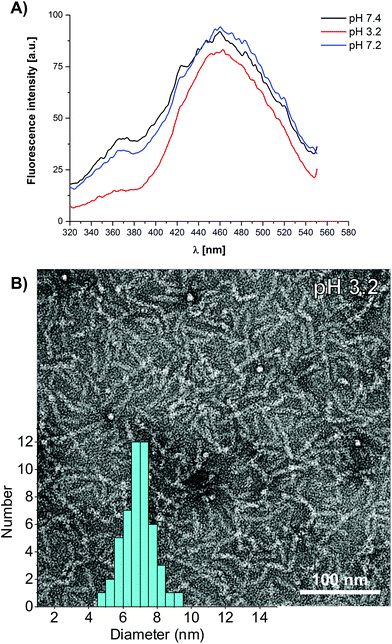
Using the aggregation induced luminescence properties of 1, we further investigated the stability of the rod-like micelles towards dilution and aimed to determine the critical aggregation concentration (CAC). The dilution of 1 in phosphate buffer (10 mM, pH 7.4) from 12.5 μM to 200 nM leads to a linear decrease of the aggregate specific emission intensity (Fig. S1†) at λem = 460 nm. It was thus not possible to determine the CAC since it is below the detection limit of our luminescence spectrometer, but we can estimate an upper limit of 200 nM. To further investigate the high stability of the self-assembled dendronised cationic amphiphile 1, we performed pH dependent photoluminescence experiments, complementary to CD spectroscopic investigations (Fig. 4A). By lowering the pH from 7.4 to pH 3.2, the aggregate specific emission band λem = 460 nm decreases, but only by 10% in intensity. The TEM images of 1 (100 μM) at pH 3.2 reveal the presence of semi-flexible nanorods with a uniform thickness of 6.8 ± 0.8 nm, thereby confirming that the assemblies are very stable and stay intact even after protonation of the imidazole groups. The addition of a base to the pH 3.2 solution of 1 restores the aggregate specific band to its original intensity at a neutral pH of 7.2 (Fig. 4A). This observation is in agreement with our tentative conclusion from the pH dependent CD data. Reversible protonation of the histidine side chain residues leads to an increased hydrophilicity and lateral swelling of the ordered core in the rod-like micelles.
To corroborate our hypothesis from the CD and luminescence spectroscopic data, we further performed encapsulation experiments with Nile Red (NR), a hydrophobic solvatochromic dye, in order to probe the polarity of the core of the rod-like micelles (Fig. S2†). When adding Nile Red (5 μM) to a solution of 1 (25 μM) in phosphate buffer at pH 7.4, we observed a fluorescence emission at λem = 621 nm (λexc = 550 nm). This value compares very well with the published studies using encapsulated NR in lipid bilayers, peptide aggregates and artificial supramolecular systems,59–65 and suggests that the dye is incorporated in an apolar microenvironment. When reducing the pH of a solution of self-assembled 1 and encapsulated NR to pH 2.8, we observed a weak bathochromic shift of 4 nm to λem = 625 nm, and a decrease in the fluorescence intensity of 4%. Both indicate that the microenvironment becomes slightly more hydrophilic but that the NR dye remains encapsulated within the assemblies. In summary, we have used a host of optical spectroscopic techniques to probe the molecular order and stability of the rod-like micelles. Hydrophobic desolvation and the β-sheet secondary structure lead to one-dimensional self-assembly, which is not switched off by protonation of the basic side chain moieties in the peptide core. In contrast to the linear oligohistidine peptide amphiphiles reported by the Stupp lab,66 a decrease in the pH therefore does not lead to a disassembly of the nanostructures, but rather swelling of the hydrophobic core.
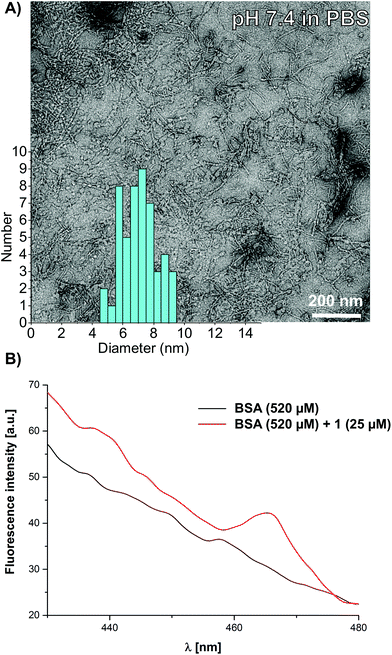
The high stability of the FHFHF based supramolecular nanostructures was investigated in physiologically relevant media, under isotonic conditions and finally also in the presence of serum albumin. A solution of 1 (100 μM) in isotonic phosphate buffered saline (PBS, 137 mM NaCl, 2.7 mM KCl, pH 7.4) was characterised using TEM. Note that we usually use Tris buffer to perform negative stain TEM in order to avoid artefacts due to phosphate salts.40,67 Nevertheless, after deposition of a solution of 1 in PBS buffer on the TEM grids, rod-like nanostructures can clearly be observed in the electron microscopy images, with an average diameter of 7.1 ± 1.1 nm (Fig. 5A). Finally we used the aggregation induced luminescence of self-assembled 1 in order to probe the stability of the assemblies in artificial serum, which is critical for applications in supramolecular drug delivery vehicles and in synthetic vaccines. Albumin accounts for approximately 60% of the proteins in blood,68 and is known to strongly effect the self-assembly of amphiphiles due to molecular crowding effects34,69 and to disrupt the formation of micelles by the absorption of single molecules.70,71 The addition of bovine serum albumin (BSA, 520 μM, 33 mg ml−1) to a solution of 1 (25 μM, 100 μg ml−1) in PBS buffer does not lead to disassembly of the aggregates, based on photoluminescence spectroscopy. The characteristic emission band of 1 monitored at λem = 460 nm was detected over the autofluorescence of the serum albumin, which was present in a 330 fold excess by weight. These findings finally confirm the excellent stability of the rod-like assemblies based on C2-symmetric peptide amphiphiles 1, even in dilute solutions and in the presence of a huge excess of BSA (Fig. 5B). We have thereby designed a very robust peptide based self-assembly building block using a 4,5-bis-(phenylthio)phthalonitrile central core that enables us to probe aggregation processes in a complex and physiologically relevant serum-like medium.
Conclusions
We have synthesised a new C2-symmetric peptide amphiphile equipped with an aggregation induced emission luminophore. An alternating phenyl alanine–histidine pentapeptide sequence FHFHF was extended with a water solubilising dendritic polycationic group and coupled to the hydrophobic 4,5-bis-(phenylthio)phthalonitrile core. In water a combination of hydrophobic shielding and β-sheet formation leads to anisotropic growth into ordered rod-like micelles. These nanostructures are remarkably stable, and the aggregation induced luminescence properties were used to estimate the upper limit for the critical aggregation concentration of <200 nM. The nanostructures are robust and stay intact in neutral and acidic buffer, in phosphate buffered saline and critically also in the presence of serum albumin. Compared to conventional design strategies we will be able to combine this new supramolecular synthon with our previously reported route for ampholytic copolymers in order to prepare highly robust, yet dynamic and tuneable pH-labile nanorods as delivery vehicles for applications in the intracellular release of cargo material.Acknowledgements
We gratefully acknowledge the support from the DFG collaborative research centre SFB 1066 [D. S. and P. B.].Notes and references
- C. Fouquey, J.-M. Lehn and A.-M. Levelut, Adv. Mater., 1990, 2, 254–257 CrossRef CAS.
- J.-M. Lehn, Angew. Chem., Int. Ed. Engl., 1990, 29, 1304–1319 CrossRef.
- R. P. Sijbesma, F. H. Beijer, L. Brunsveld, B. J. B. Folmer, K. J. H. K. Hirschberg, R. F. M. Lange, J. K. L. Lowe and E. W. Meijer, Science, 1997, 278, 1601–1604 CrossRef CAS PubMed.
- L. Brunsveld, B. J. B. Folmer, E. W. Meijer and R. P. Sijbesma, Chem. Rev., 2001, 101, 4071–4097 CrossRef CAS PubMed.
- D. Zhao and J. S. Moore, Org. Biomol. Chem., 2003, 1, 3471–3491 CAS.
- T. F. A. de Greef, M. M. J. Smulders, M. Wolffs, A. P. H. J. Schenning, R. P. Sijbesma and E. W. Meijer, Chem. Rev., 2009, 109, 5687–5754 CrossRef CAS PubMed.
- Z. Chen, A. Lohr, C. R. Saha-Möller and F. Würthner, Chem. Soc. Rev., 2009, 38, 564–584 RSC.
- C. Rest, R. Kandanelli and G. Fernandez, Chem. Soc. Rev., 2015, 44, 2543–2572 RSC.
- E. Krieg, M. M. C. Bastings, P. Besenius and B. Rybtchinski, Chem. Rev., 2016, 116, 2414–2477 CrossRef CAS PubMed.
- T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813–817 CrossRef CAS PubMed.
- R. Chapman, M. Danial, M. L. Koh, K. A. Jolliffe and S. Perrier, Chem. Soc. Rev., 2012, 41, 6023–6041 RSC.
- J. Montenegro, M. R. Ghadiri and J. R. Granja, Acc. Chem. Res., 2013, 46, 2955–2965 CrossRef CAS PubMed.
- J. D. Hartgerink, E. Beniash and S. I. Stupp, Science, 2001, 294, 1684–1688 CrossRef CAS PubMed.
- J. D. Hartgerink, E. Beniash and S. I. Stupp, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 5133–5138 CrossRef CAS PubMed.
- G. A. Silva, C. Czeisler, K. L. Niece, E. Beniash, D. A. Harrington, J. A. Kessler and S. I. Stupp, Science, 2004, 303, 1352–1355 CrossRef CAS PubMed.
- A. M. Smith, R. J. Williams, C. Tang, P. Coppo, R. F. Collins, M. L. Turner, A. Saiani and R. V. Ulijn, Adv. Mater., 2008, 20, 37–41 CrossRef CAS.
- S. Fleming and R. V. Ulijn, Chem. Soc. Rev., 2014, 43, 8150–8177 RSC.
- V. Percec, A. E. Dulcey, M. Peterca, M. Ilies, M. J. Sienkowska and P. A. Heiney, J. Am. Chem. Soc., 2005, 127, 17902–17909 CrossRef CAS PubMed.
- H. Frisch, J. P. Unsleber, D. Lüdeker, M. Peterlechner, G. Brunklaus, M. Waller and P. Besenius, Angew. Chem., Int. Ed., 2013, 52, 10097–10101 CrossRef CAS PubMed.
- K. Petkau-Milroy and L. Brunsveld, Org. Biomol. Chem., 2013, 11, 219–232 CAS.
- Y. Bae, S. Fukushima, A. Harada and K. Kataoka, Angew. Chem., Int. Ed., 2003, 42, 4640–4643 CrossRef CAS PubMed.
- A. K. H. Hirsch, F. Diederich, M. Antonietti and H. G. Börner, Soft Matter, 2010, 6, 88–91 RSC.
- J. Shi, A. R. Votruba, O. C. Farokhzad and R. Langer, Nano Lett., 2010, 10, 3223–3230 CrossRef CAS PubMed.
- A. G. Cheetham, P. Zhang, Y.-A. Lin, L. L. Lock and H. Cui, J. Am. Chem. Soc., 2013, 135 Search PubMed.
- K. Miyata, N. Nishiyama and K. Kataoka, Chem. Soc. Rev., 2012, 41, 2562–2574 RSC.
- J. A. Hubbell and A. Chilkoti, Science, 2012, 337, 303–305 CrossRef PubMed.
- J. Nicolas, S. Mura, D. Brambilla, N. Mackiewicz and P. Couvreur, Chem. Soc. Rev., 2013, 42, 1147–1235 RSC.
- M. Talelli, M. Barz, C. J. F. Rijcken, F. Kiessling, W. E. Hennink and T. Lammers, Nano Today, 2015, 10, 93–117 CrossRef CAS PubMed.
- J. S. Rudra, Y. F. Tian, J. P. Jung and J. H. Collier, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 622–627 CrossRef CAS PubMed.
- J. S. Rudra, T. Sun, K. C. Bird, M. D. Daniels, J. Z. Gasiorowski, A. S. Chong and J. H. Collier, ACS Nano, 2012, 6, 1557–1564 CrossRef CAS PubMed.
- M. A. Swartz, S. Hirosue and J. A. Hubbell, Sci. Transl. Med., 2012, 4, 148rv149 Search PubMed.
- L. Nuhn, S. Hartmann, B. Palitzsch, B. Gerlitzki, E. Schmitt, R. Zentel and H. Kunz, Angew. Chem., Int. Ed., 2013, 52, 10652–10656 CrossRef CAS PubMed.
- S. R. Bull, M. O. Guler, R. E. Bras, T. J. Meade and S. I. Stupp, Nano Lett., 2004, 5, 1–4 CrossRef PubMed.
- P. Besenius, J. L. M. Heynens, R. Straathof, M. M. L. Nieuwenhuizen, P. H. H. Bomans, E. Terreno, S. Aime, G. J. Strijkers, K. Nicolay and E. W. Meijer, Contrast Media Mol. Imaging, 2012, 7, 356–361 CrossRef CAS PubMed.
- P. Y. W. Dankers and E. W. Meijer, Bull. Chem. Soc. Jpn., 2007, 80, 2047–2073 CrossRef CAS.
- A. Mata, Y. Geng, K. J. Henrikson, C. Aparicio, S. R. Stock, R. L. Satcher and S. I. Stupp, Biomaterials, 2010, 31, 6004–6012 CrossRef CAS PubMed.
- J. B. Matson and S. I. Stupp, Chem. Commun., 2012, 48, 26–33 RSC.
- M. J. Webber, E. A. Appel, E. W. Meijer and R. Langer, Nat. Mater., 2016, 15, 13–26 CrossRef CAS PubMed.
- M. von Gröning, I. de Feijter, M. C. A. Stuart, I. K. Voets and P. Besenius, J. Mater. Chem. B, 2013, 1, 2008–2012 RSC.
- H. Frisch, Y. Nie, S. Raunser and P. Besenius, Chem. – Eur. J., 2015, 21, 3304–3309 CrossRef CAS PubMed.
- R. Appel, S. Tacke, J. Klingauf and P. Besenius, Org. Biomol. Chem., 2015, 13, 1030–1039 CAS.
- R. Appel, J. Fuchs, S. M. Tyrrell, P. A. Korevaar, M. C. A. Stuart, I. K. Voets, M. Schönhoff and P. Besenius, Chem. – Eur. J., 2015, 21, 19257–19264 CrossRef CAS PubMed.
- B. Kemper, Y. R. Hristova, S. Tacke, L. Stegemann, L. S. van Bezouwen, M. C. A. Stuart, J. Klingauf, C. A. Strassert and P. Besenius, Chem. Commun., 2015, 51, 5253–5256 RSC.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC.
- B.-K. An, S.-K. Kwon, S.-D. Jung and S. Y. Park, J. Am. Chem. Soc., 2002, 124, 14410–14415 CrossRef CAS PubMed.
- M. K. Müller and L. Brunsveld, Angew. Chem., Int. Ed., 2009, 48, 2921–2924 CrossRef PubMed.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- B. Xu, M. Xie, J. He, B. Xu, Z. Chi, W. Tian, L. Jiang, F. Zhao, S. Liu, Y. Zhang, Z. Xu and J. Xu, Chem. Commun., 2013, 49, 273–275 RSC.
- J. Mei, Y. Hong, J. W. Y. Lam, A. Qin, Y. Tang and B. Z. Tang, Adv. Mater., 2014, 26, 5429–5479 CrossRef CAS PubMed.
- D. Ding, K. Li, B. Liu and B. Z. Tang, Acc. Chem. Res., 2013, 46, 2441–2453 CrossRef CAS PubMed.
- K. Petkau-Milroy, M. H. Sonntag, A. H. A. M. van Onzen and L. Brunsveld, J. Am. Chem. Soc., 2012, 134, 8086–8089 CrossRef CAS PubMed.
- P. Ahlers, H. Frisch and P. Besenius, Polym. Chem., 2015, 6, 7245–7250 RSC.
- S. Brahms, J. Brahms, G. Spach and A. Brack, Proc. Natl. Acad. Sci. U. S. A., 1977, 74, 3208–3212 CrossRef CAS.
- S. E. Paramonov, H.-W. Jun and J. D. Hartgerink, J. Am. Chem. Soc., 2006, 128, 7291–7298 CrossRef CAS PubMed.
- H. Jiang, M. O. Guler and S. I. Stupp, Soft Matter, 2007, 3, 454–462 RSC.
- P. Besenius, K. P. van den Hout, H. M. H. G. Albers, T. F. A. de Greef, L. L. C. Olijve, T. M. Hermans, B. F. M. de Waal, P. H. H. Bomans, N. A. J. M. Sommerdijk, G. Portale, A. R. A. Palmans, M. H. P. van Genderen, J. A. J. M. Vekemans and E. W. Meijer, Chem. – Eur. J., 2011, 17, 5193–5203 CrossRef CAS PubMed.
- P. Besenius, G. Portale, P. H. H. Bomans, H. M. Janssen, A. R. A. Palmans and E. W. Meijer, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 17888–17893 CrossRef CAS PubMed.
- W. Rettig, Angew. Chem., Int. Ed. Engl., 1986, 25, 971–988 CrossRef.
- P. Greenspan and S. D. Fowler, J. Lipid Res., 1985, 26, 781–789 CAS.
- M. Sutter, S. Oliveira, N. N. Sanders, B. Lucas, A. Hoek, M. A. Hink, A. J. W. G. Visser, S. C. Smedt, W. E. Hennink and W. Jiskoot, J. Fluoresc., 2007, 17, 181–192 CrossRef CAS PubMed.
- B. Demeule, R. Gurny and T. Arvinte, Int. J. Pharm., 2007, 329, 37–45 CrossRef CAS PubMed.
- A. Hawe, M. Sutter and W. Jiskoot, Pharm. Res., 2008, 25, 1487–1499 CrossRef CAS PubMed.
- R. Mishra, D. Sjolander and P. Hammarstrom, Mol. BioSyst., 2011, 7, 1232–1240 RSC.
- J. Boekhoven, A. M. Brizard, P. van Rijn, M. C. A. Stuart, R. Eelkema and J. H. van Esch, Angew. Chem., Int. Ed., 2011, 50, 12285–12289 CrossRef CAS PubMed.
- I. de Feijter, P. Besenius, L. Albertazzi, E. W. Meijer, A. R. A. Palmans and I. K. Voets, Soft Matter, 2013, 9, 10025–10030 RSC.
- T. J. Moyer, J. A. Finbloom, F. Chen, D. J. Toft, V. L. Cryns and S. I. Stupp, J. Am. Chem. Soc., 2014, 136, 14746–14752 CrossRef CAS PubMed.
- M. Ohi, Y. Li, Y. Cheng and T. Walz, Biol. Proced. Online, 2004, 6, 23–34 CrossRef CAS PubMed.
- H. A. Krebs, Annu. Rev. Biochem., 1950, 19, 409–430 CrossRef CAS PubMed.
- A. Ghosh, C. J. Buettner, A. A. Manos, A. J. Wallace, M. F. Tweedle and J. E. Goldberger, Biomacromolecules, 2014, 15, 4488–4494 CrossRef CAS PubMed.
- H. Chen, S. Kim, W. He, H. Wang, P. S. Low, K. Park and J.-X. Cheng, Langmuir, 2008, 24, 5213–5217 CrossRef CAS PubMed.
- J. Lu, S. C. Owen and M. S. Shoichet, Macromolecules, 2011, 44, 6002–6008 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional Fig. S1–S6, detailed Experimental section: materials, synthesis, characterisation, analysis, methods and instrumentation. See DOI: 10.1039/c6ob00292g |
| ‡ In methanol we observe an intensity weighted average fluorescence lifetime τ = 3 ns, which is typical of small organic fluorophores (Fig. S3–S6†). |
| This journal is © The Royal Society of Chemistry 2016 |