Some chemical speculation on the biosynthesis of corallidictyals A–D†
Abstract
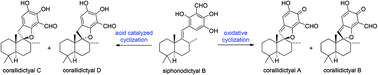
The efficient conversion of siphonodictyal B into the spirocyclic natural products corallidictyals A–D has been achieved via oxidative and acid catalyzed cyclizations. The oxidative cyclization of siphonodictyal B occured spontaneously under aerobic oxidation conditions, which suggests that corallidictyals A and B are possibly artefacts of the isolation process. The mechanism of the oxidative cyclization of siphonodictyal B could be described as either an anionic 5-endo-trig cyclization (which is formally disfavoured by Baldwin's rules), or as an electrocyclic reaction, of an ortho-quinone intermediate.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...