Facile synthesis of enantioenriched phenol-sulfoxides and their aluminum complexes†
Abstract
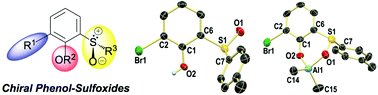
Chiral phenolic p-tolylsulfoxides and t-butylsulfoxides were prepared by several short synthetic routes starting from readily available starting materials. The key synthetic step was the reaction of lithiated arenes with menthyl sulfinates or enantioselective oxidation of a t-butyl sulfide. Well-defined neutral ligand–AlMe2 complexes were obtained by stoichiometric treatment with AlMe3.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...