Fluorine-directed 1,2-trans glycosylation of rare sugars†
Abstract
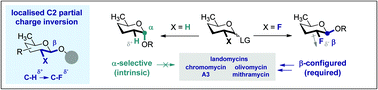
To reconcile the urgent need to access well defined β-configured 2,6-di-deoxypyranose analogues for chemical biology, with the intrinsic α-selectivity of the native system, the directing role of fluorine at C2 has been explored. Localised partial charge inversion (C–Hδ+ → C–Fδ−) elicits a reversal of the substrate-based α-stereoselectivity, irrespective of the protecting group electronics.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...